- Title
-
Autophosphorylation and Pin1 binding coordinate DNA damage-induced HIPK2 activation and cell death
- Authors
- Bitomsky, N., Conrad, E., Moritz, C., Polonio-Vallon, T., Sombroek, D., Schultheiss, K., Glas, C., Greiner, V., Herbel, C., Mantovani, F., Del Sal, G., Peri, F., and Hofmann, T.G.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
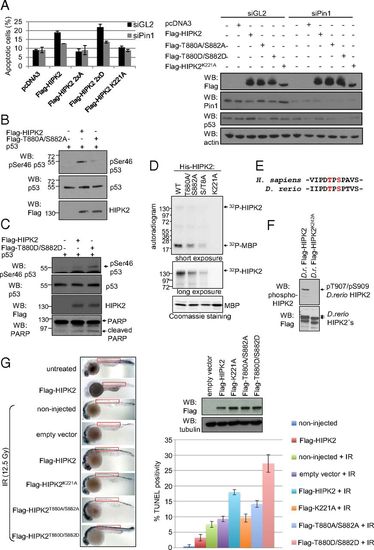
HIPK2 autophosphorylation at Thr880/Ser882 regulates its apoptotic function in human cells and zebrafish embryos. (A) HIPK2 autophosphorylation is critical for its apoptotic function. HCT116 cells were transfected with empty vectors and HIPK2 expression vectors as indicated. Cells were analyzed by FACS for apoptosis-associated annexin V-positivity after annexin V/propidium iodide staining. The graph shows means and SD. n = 3. siGL indicates control siRNA transfected cells (black bars); siPin1 indicates cells depleted of Pin1 (gray bars). Flag-HIPK2, Pin1, and p53 levels were determined by immunoblotting. A representative result is shown. (B and C) HIPK2 autophosphorylation at Thr880/Ser882 regulates p53 Ser46 phosphorylation capacity. H1299 cells were transfected with the expression vectors indicated, and cell lysates were analyzed by immunoblotting. (D) HIPK2 autophosphorylation correlates with its kinase activity. Bacterially expressed 6xHis-HIPK2 proteins were used for radioactive in vitro kinase assay with MBP as substrate and analyzed by autoradiography. Input was controlled by Coomassie brilliant blue staining and HIPK2 immunoblotting. Input levels of the His-HIPK2 proteins are shown in Fig. 1C, which was performed in parallel to this experiment here by using the same batch and amounts of His-HIPK2 proteins. (E) The HIPK2 autophosphorylation site is conserved between zebrafish and humans. (F) D. rerio HIPK2 is autophosphorylated. H1299 cells were transfected with expression vectors coding for wild-type or kinase-deficient zebrafish HIPK2. Total cell extracts were analyzed by immunoblotting using the indicated antibodies. (G) HIPK2 autophosphorylation at T880/S882 potentiates apoptosis induction in zebrafish embryos. Embyos were microinjected with mRNAs coding for HIPK2 proteins as indicated, exposed to IR (12.5 Gy), and analyzed and quantified for TUNEL positivity. |
|
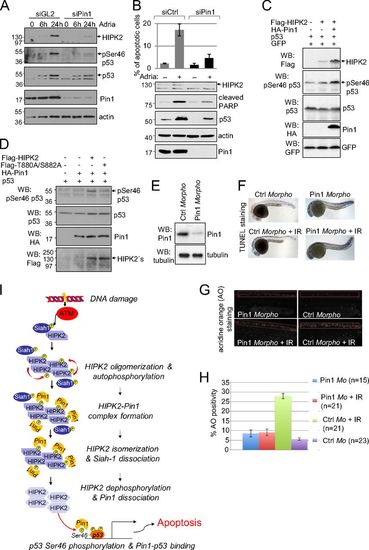
Pin1 regulates HIPK2-mediated p53 Ser46 phosphorylation in human cells and is required for DNA damage-induced apoptosis in zebrafish. (A) Pin1 knockdown inhibits Adriamycin-induced HIPK2 stabilization and p53 Ser46 phosphorylation. HCT116 cells were transfected with control (GL2, luciferase) or Pin1-specific siRNA as indicated and treated with 1 μg/mL Adriamycin. Cells were lysed at the timepoints indicated and analyzed by immunoblotting. (B) Pin1 depletion impairs apoptosis induction. HCT116 cells were transfected with Pin1-specific or control siRNAs. Cells were treated 24 h with 1 μg/mL Adriamycin. Sub-G1 DNA content analysis was performed by propidium-iodide staining and FACS analysis. The graph shows means and SD from three independent experiments. Knockdown efficiency and apoptosis induction was controlled by immunoblotting. (C) Pin1 potentiates HIPK2-mediated p53 Ser46 phosphorylation. H1299 cells were transfected as indicated. Cell lysates were analyzed by immunoblotting. (D) Pin1–HIPK2 interaction seems to be important for induction of p53 Ser46 phosphorylation by Pin1. H1299 cells were transfected with the expression vectors indicated. Amounts of transfected HIPK2 expression vectors were adjusted to result in a comparable HIPK2 expression. Cell lysates were analyzed by immunoblotting. (E–H) Pin1 depletion in zebrafish embryos results in decreased apoptosis induction upon IR in vivo. (E) Zebrafish eggs were injected with Pin1-specific or control morpholinos. Knockdown of endogenous Pin1 was controlled by immunoblotting of whole-body embryo lysates. (F and G) At 24 h after fertilization (hpf) embryos were exposed to 12.5 Gy IR and analyzed 30 hpf for apoptosis induction by using either TUNEL (F) or acridine orange (AO) (G) staining. Representative images are shown. (H) Quantification of the AO staining in the zebrafish embryos. (A–G) Representative experiments are shown. (I) Proposed mechanism of HIPK2 activation upon DNA damage. For details, see Discussion. PHENOTYPE:
|
|
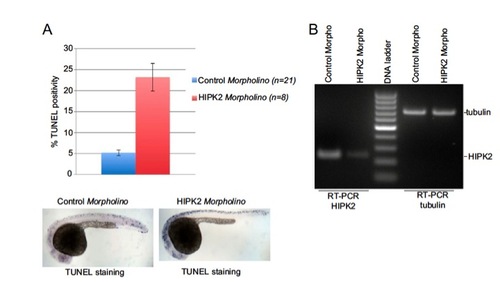
HIPK2 depletion in zebrafish embryos. HIPK2 depletion in zebrafish embryos results in spontaneous induction of apoptosis. (A) HIPK2 morpholino and control morpholino-injected embryos where analyzed by TUNEL staining. Quantification of TUNEL positivity and representative images are shown. (B) HIPK2 and tubulin mRNA levels in zebrafish embryos were analyzed by RT-PCR analysis. PHENOTYPE:
|