- Title
-
Genetic defects of GDF6 in the zebrafish out of sight mutant and in human eye developmental anomalies
- Authors
- den Hollander, A.I., Biyanwila, J., Kovach, P., Bardakjian, T., Traboulsi, E., Ragge, N.K., Schneider, A., and Malicki, J.
- Source
- Full text @ BMC Genet.
|
Phenotype of the zebrafish out of sight mutant. (A - B) Lateral views of wild-type zebrafish (A) and of the outm233 mutant (B) at 36 hpf, 48 hpf (a1 and b1), 72 hpf (a2 and b2) and 96 hpf (a3 and b3). The outm233 mutant is characterized by a severe reduction in eye size. No obvious defects are observed in other organs. (C - D) Transverse plastic sections of wild-type (C) and outm233 mutant (D) retinae at 36 hpf, 4 dpf (c1 and d1) and 5 dpf (c2 and d2). Although the retina and lens are grossly reduced in size in outm233 mutants, their overall architectures appear largely intact. (E - F) An anophthalmic outm233 homozygous mutant at ca. 2 months of age. An enlargement of the head region is shown in (F). (G - H) A wild-type individual (G) and its microphthalmic outm233 sibling (H) at 2.5 months of age. In A, B, and E-H, anterior is to the left PHENOTYPE:
|
|
Positional cloning of the zebrafish out of sight mutation. (A) The out locus maps to chromosome 16 between markers Z6293 and Z8819. Sequence analysis of the gdf6a gene from this interval identified a mutation affecting its start codon (c.1A > G, p. Met1Val) in mutant (C) but not wild-type (B) individuals. SP = signal peptide, ASD = active signaling domain. (D, E) The overexpression of wild-type (E) but not mutant (D) gdf6a causes ventral eye defects. Shown are lateral (left panels) and ventro-lateral (right panels) views of zebrafish larvae at 3 dpf. Anterior is to the left, asterisks indicate the lens. (F) The frequency of ventral eye defects, following the overexpression of wild-type or mutant gdf6a in four independent experiments, as indicated on the horizontal axis (1 through 4). Numbers above the bar graph indicate sample sizes. PHENOTYPE:
|
|
The number of cells in wild-type and outm233mutant retinae. (A - B) Hoechst staining of 4-μm plastic sections through the retina of wild-type (A) and outm233 (B) embryos at 36 hpf. (C) Diagram showing the number of nuclei that were counted in the dorsal, ventral, and entire retina. At 36 hpf, cell numbers in mutant embryos are not significantly different from these in wild-type siblings. Cells were counted on sections from 7 and 4 embryos for wild-type and mutant, respectively. (D - E) Hoechst staining of 4-μm plastic sections through the retina of wild-type (D) and outm233 (E) embryos at 96 hpf. (F) Diagram showing the number of nuclei that were counted in the ganglion cell layer (GCL), in the inner nuclear layer (INL) and in the photoreceptor layer (PRCL). By 96 hpf, cell numbers in all these three layers are reduced in mutant embryos, compared to the wild type (for GCL, p < 0.01; for INL and PRCL, p < 0.001; t-test). For both wild-type and mutant, cells were counted on sections from 6 embryos. Asterisks indicate the lens, and arrowheads the optic nerve. |
|
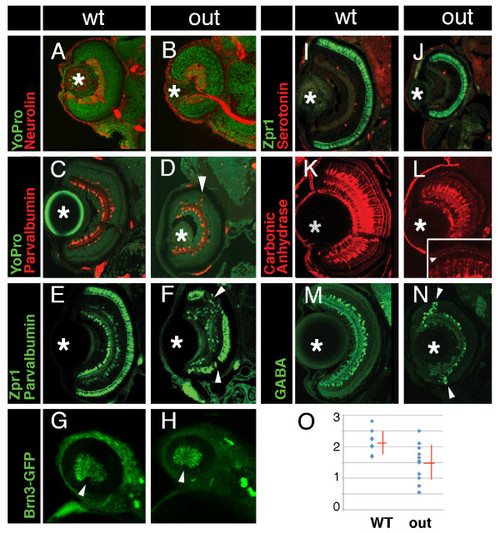
Cell fate in wild-type and outm233 retinae. (A-B) Transverse sections through the retina stained with anti-neurolin antibody (red) in wild-type (A) and outm233 (B) embryos. The ganglion cell layer and the optic nerve display grossly normal appearance at 56 hpf. Sections were counterstained with YoPro (green). (C-D). Anti-parvalbumin antibody (red) stains a subset of amacrine cells on transverse sections through the retinae of wild-type (C) and outm233 mutant (D) embryos. A displacement of some amacrine cells towards and occasionally into the photoreceptor cell layer is observed in approximately 50% of outm233 embryos (arrowhead in D). (E - F) Anti-paravalbumin and Zpr1 double immunostaining (both in green) in wild-type (E) and in outm233 mutant (F) embryos. In approximately 50% of the embryos, Zpr1-positive cells are absent in the dorsal and/or ventral regions of the photoreceptor layer (arrowheads in F). (G - H) Images of whole embryos at 42.5 hpf. Ganglion cells are visualized with brn3c-GFP transgene in wild-type (G) and outm233 mutant (H) embryos. Anterior is to the left. (I -J) The Zpr1 antibody stains red and green cones (green signal) while anti-serotonin antibody labels serotonin-positive amacrine cells (red) in wild-type (I) and outm233 mutant (J) embryos. (K-L) Anti-carbonic anhydrase antibody (red) stains Müller glia in wild-type (K) and outm233 mutant (L) embryos. Arrowhead in the inset indicates the apical processes of the Muller glia, which terminate at the outer limiting membrane. (M - N) Anti-GABA antibody (green) stains a subset of amacrine cells in wild-type (M) and outm233 mutant (N) embryos. On the majority of sections, the number of GABA-positive neurons is increased in the retinal periphery of outm233 homozygotes (arrowheads). (O) Graph showing the frequency of GABA-positive cells in the central retina. The number of cells per an arbitrary unit of distance if provided. Fewer GABA-positive cells are observed in the mutant, compared to the wild type (p < 0.01, t-test). Blue dots represent sections. As some sections have the same frequency of GABA-positive cells, the number of dots does not equal the number of sections. n ≥ 4 embryos for both wild-type and mutant. Asterisks mark the lens. (C-F) and (I-N) show retinae at 5 dpf. PHENOTYPE:
|
|
Cell proliferation in the out of sight retina. (A - B) Immunolabeling of transverse cryosections through the retinae of wild-type (A) and outm233 mutant (B) embryos with anti-phospho-histone H3 (red) antibodies at 36 hpf. Sections were counterstained with YoPro (green). (C) Graph illustrating the numbers of phospho-H3 histone-positive nuclei on sections through wild-type and outm233 mutant retinae as indicated. (D) Graph showing the ratio of phospho-H3-positive cells to all cells. No differences between wild-type and outm233 embryos were identified. In (C - D), black dots represent sections. As some sections have the same number of phospho-H3-positive cells, the number of dots does not equal the number of sections. n ≥ 10 embryos and ≥ 15 sections for both wild-type and mutant samples. Asterisks indicate the lens, and arrowheads the optic nerve. Dorsal is up. |
|
Apoptosis in outm233 mutant embryos. (A - H) Transverse cryosections through the zebrafish eye. Apoptosis is detected using the TUNEL assay (red signal). Levels of apoptosis are abnormally high in outm233 mutant (E-H) eyes during early neurogenesis. At 30 hpf, there is an increase of apoptosis in the lens and the retina of mutants (E) compared to the wild-type (A). Apoptosis persists in and around the lens region at 48 hpf (F, compare to B). By 76 hpf, hardly any apoptosis is visible in the mutant eye (G), and a significantly increased amount of apoptosis occurs in the wild-type (C) retina, compared to the mutant. At 5 dpf, hardly any apoptotic cells are found in both the wild type (D) and the mutant (H). (I) The amount of cell death is quantitated. The average number of apoptotic cells per section is provided. Arrowheads point to the lens. n ≥ 9 sections from at least 3 embryos for each time point both for wild-type and mutant samples. |