- Title
-
Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death
- Authors
- Owens, K.N., Santos, F., Roberts, B., Linbo, T., Coffin, A.B., Knisely, A.J., Simon, J.A., Rubel, E.W., and Raible, D.W.
- Source
- Full text @ PLoS Genet.
|
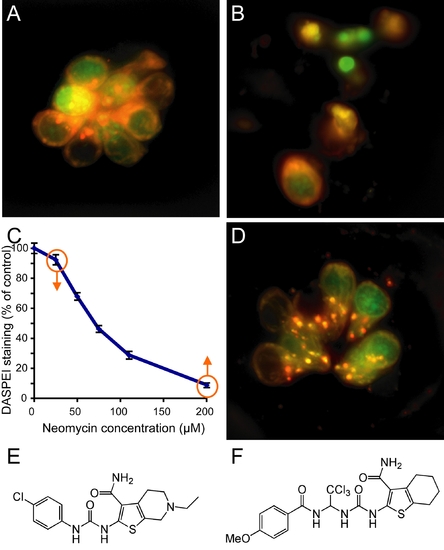
Screening for modifiers of aminoglycoside toxicity. |
|
Mutations that confer protection against neomycin exposure. PHENOTYPE:
|
|
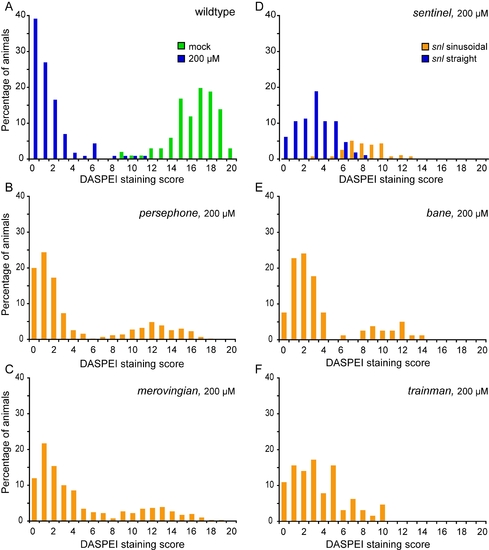
Hair cell retention after neomycin treatment in wildtype and mutant animals. PHENOTYPE:
|
|
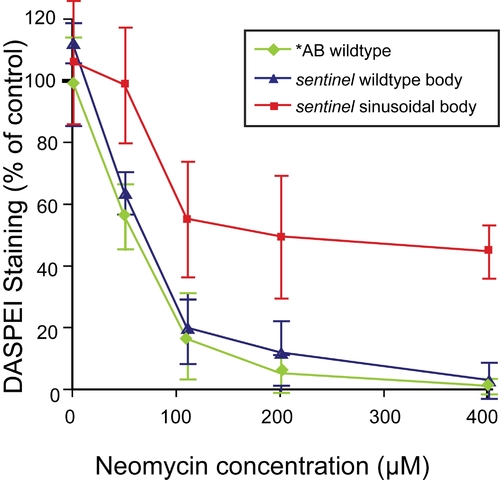
Dose dependent protection of sentinel mutants to neomycin. PHENOTYPE:
|
|
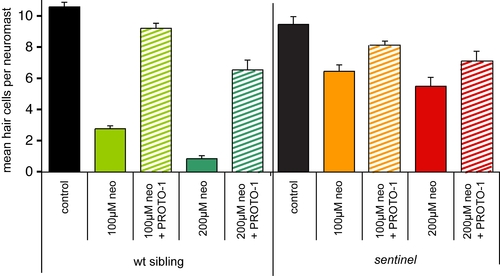
Epistasis analysis of sentinel and protective compounds. PHENOTYPE:
|
|
sentinel mutation does not affect transduction-dependent dye or aminoglycoside uptake. |
|
sentinel mutation and PROTO-1 do not protect against cisplatin. PHENOTYPE:
|
|
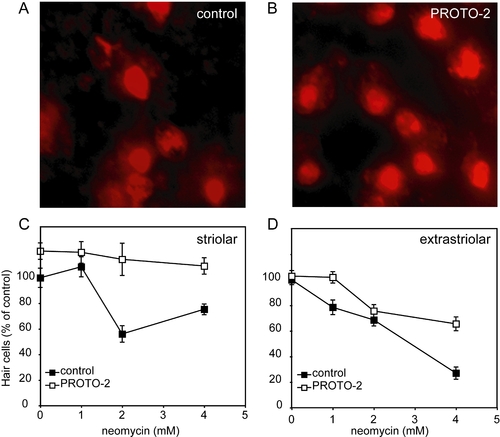
Protective compounds reduce neomycin toxicity in adult mouse utricle cultures. |
|
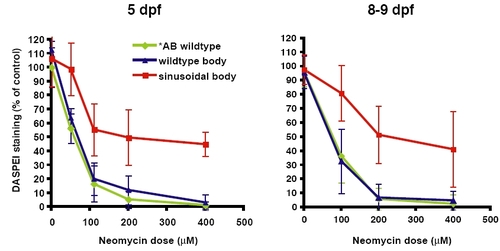
Comparison of the dose response curve of 5 dpf versus 8–9 dpf sentinel mutants. Hair cell staining by DASPEI was assessed after neomycin exposure among progeny of sentinel heterozygous parents with wildtype body shape (blue) or sinusoidal body shape (red). The dose response curves of wildtype *AB control fish are shown (green). (A) Dose-response at 5 dpf. (B) Dose-response at 8–9 dpf. Error bars are ±1 S.D. |
|
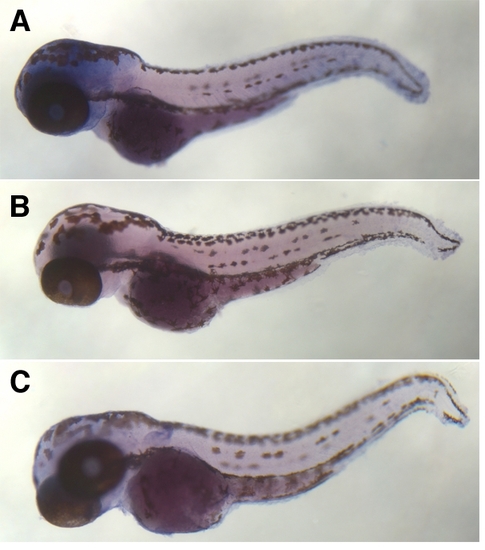
In situ hybridization of biotinylated probes to the sentinel locus reveals ubiquitous expression. (A) Wildtype *AB larvae 64 hpf, antisense probe. (B) *AB larvae 64 hpf, sense probe. (C) sentinel mutants 64 hpf, antisense probe. EXPRESSION / LABELING:
|
|
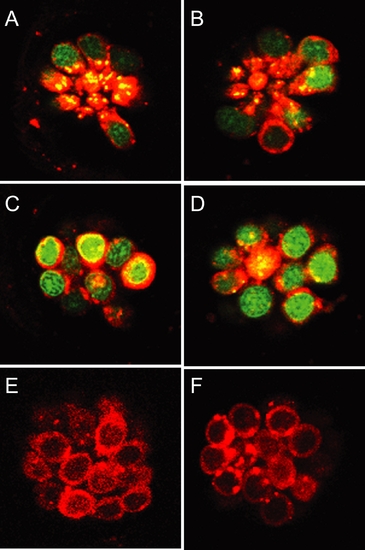
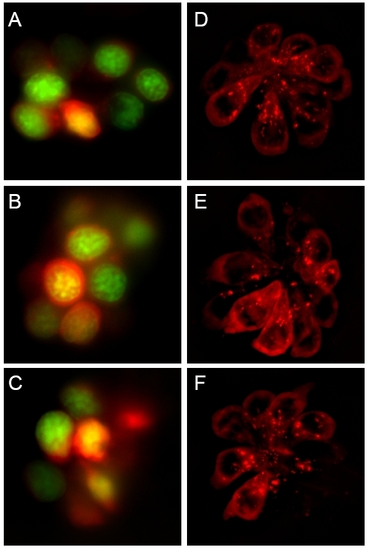
Protective compounds do not affect transduction-dependent dye or aminoglycoside uptake. (A-C) Rapid entry (45 sec) of 3 μM FM 1–43 (red) into untreated (A), PROTO-1 (B), or PROTO-2 (C) treated hair cells. Nuclei are labeled with Yo-Pro-1 (green). (D-F) Exposure to gentamicin-conjugated Texas Red results in rapid labeling of untreated (D), 10 μM PROTO-1 (E), or PROTO-2 (F) pretreated hair cells. |

Unillustrated author statements PHENOTYPE:
|