- Title
-
Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration
- Authors
- Lee, Y., Grill, S., Sanchez, A., Murphy-Ryan, M., Poss, K.D.
- Source
- Full text @ Development
|
Amputated zebrafish caudal fins display position-dependent rates of regenerative growth. (A) Appearance of the zebrafish fin immediately following a double amputation surgery, with the injured portion at the top of the image. The amputation planes are indicated by arrows (black, proximal; red, distal), and asterisks mark lateral rays 2 and 3 that are compared in this study. (B) Only 4 days after amputation (dpa; assessed at 33°C), the fin has regenerated a significant number of lost structures. The ventral lobe of the fin (left), after a more proximal amputation, is regenerating more rapidly than the right, dorsal lobe. (C) By 7 dpa, the ventral regenerate has reached nearly the same PD level as the dorsal regenerate. (D) Growth is greater after proximal amputations compared with distal amputations throughout regeneration (mean±s.e.m.; *P<0.05, t-test). (E) Growth rate is greater after proximal amputations than distal throughout regeneration. |
|
Blastemal length and mitotic index depend on PD position. (A,B) Proximal and distal 3 dpa regenerates (33°C) of the same fin stained for BrdU incorporation (fins collected 30 minutes post-injection). The proximal regenerate has a greater PD length of especially BrdU-dense blastemal mesenchyme (brackets). (C,D) Proximal and distal 3 dpa regenerates of the same fin stained for phosphorylated Histone-3 (H3P), an indicator of mitosis. Fine points indicate individual mesenchymal mitotic nuclei. This particular fin was chosen because even though it has similar blastemal sizes for the proximal and distal regenerates (brackets; actual BrdU stain is not shown), there are clearly more H3P-positive cells in the proximal blastema. As reported previously, fins show non-specific epidermal fluorescence at the distal edge of the regenerate (Poss et al., 2002b). (E) Quantification of blastemal length and mitotic index at 3 dpa (*P<0.05, t-test). |
|
Amputation level controls the amount of Fgf target gene expression during regeneration. In situ hybridization analysis of Fgf target gene expression in 3 dpa double-amputated fins (33°C). (A) A representative section from a single-amputated fin demonstrates expression for mkp3, sef and spry4 in both the basal epidermal layer and the distal region of the blastema (arrowheads indicate in situ hybridization signals). (B) Whole-mount images for each gene show proximal and distal regenerates of the same double-amputated fin. mkp3 (15 out of 24 regenerates), sef (8 out of 11 regenerates) and spry4 (12 out of 18 regenerates) were usually expressed more strongly in proximal regenerates than distal. We never detected greater expression of these genes in distal regenerates. EXPRESSION / LABELING:
|
|
Position-dependent length of Fgf target gene expression domains. (A,B) Images from the same double-amputated fin regenerate demonstrates a longer PD length of mkp3 expression (asterisks, brackets) in proximal regenerates. (C) The length of the proximal signal was 28% longer than the distal signal on average (n=8; *P<0.005, t-test). (D-F) 3 dpa fin regenerate (33°C) stained for mkp3 expression (D) and BrdU incorporation (E). Cells in the distal blastema and basal epidermal layers expressing mkp3 show little proliferation. However, proliferative blastemal mesenchyme is bordered by epidermal mkp3 expression/Fgf signaling (F). EXPRESSION / LABELING:
|
|
Transgenic fish that facilitate inducible expression of a dominant-negative Fgfr1 construct. (A,B) hsp70:dn-fgfr1 transgenic and wild-type embryos were raised until sphere stage at 28°C, shifted to 37°C for 1 hour, and returned to 28°C until 28 hpf. Transgenic embryos are truncated and display Egfp fluorescence (arrowheads). (C,D) Section through a wild-type (C) or hsp70:dn-fgfr1 (D) 4 dpa fin regenerate 5 hours after heat induction. Strong Egfp fluorescence is observed in all cells of the transgenic regenerate, including the fgfr1/mkp3/sef/spry4-positive basal epidermal layer (arrowhead). Nuclei are stained with DAPI (blue). (E) Whole-mount image of fin shown in D, demonstrating Egfp fluorescence throughout the fin. The regenerate shows stronger fluorescence than the non-regenerating portion at 4 dpa. This is probably due in part to the scarceness of pigment cells and differentiated bone in the newly formed tissue that might impede fluorescence detection. (F,G) Adult fin regeneration is blocked by daily heat-induction of dn-fgfr1 at 38°C. Wild-type (F) and hsp70:dn-fgfr1 (G) fins are shown at 7 dpa. |
|
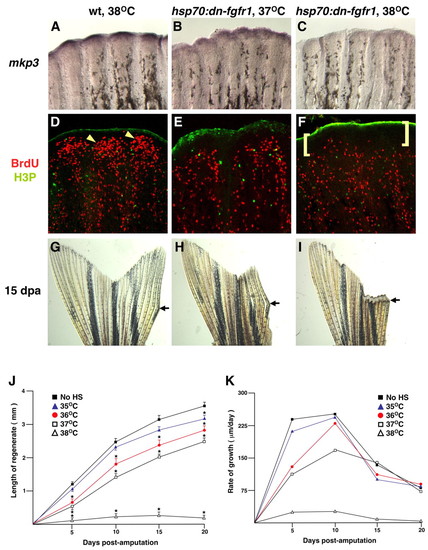
Experimental attenuation of Fgf signaling alters regenerative proliferation and growth in a dose-dependent manner. (A-C) Images of 4 dpa fin regenerates (26°C) that were heat-induced once at the indicated temperature and collected and examined for mkp3 expression 5 hours later (violet stain). mkp3 expression is greatest in wild types treated at 38°C (A), less in hsp70:dn-fgfr1 transgenics treated at 37°C (B), and undetectable in transgenics given a strong 38°C induction (C). (D-F) Animals treated in the same way as those in A-C, respectively, were assessed for BrdU incorporation and H3P staining. Blastemal BrdU-labeling density (arrowheads in D) is reduced by the 37°C shock in transgenics (E) and still further by the 38°C shock (F). Brackets in (F) indicate a region of Fgf-dependent proliferation. (G-I) Images of 15 dpa fin regenerates given a daily heat induction; to highlight the extent of regeneration, only the right lobe was amputated. Arrows indicate points of amputation. Wild-type fins induced at 38°C or uninduced transgenic fins regenerated normally (G), those induced at 37°C displayed partial regeneration (H), and those induced at 38°C showed a complete block (I). (J,K) Animals were induced daily at four different temperatures, or had no induction, and regenerative growth was measured at 5, 10, 15 and 20 dpa. Regenerative rates were calculated based on these numbers. A daily heat induction to 37°C nearly halves the rate of regeneration at 5 dpa; daily induction to 38°C blocks regeneration (n=5; * P<0.05, significantly different from no HS, t-test). EXPRESSION / LABELING:
|
|
Regenerative growth in distal fin regenerates is more sensitive to partial Fgfr inhibition than proximal regenerative growth. (A) Daily exposure of double-amputated hsp70:dn-fgfr1 zebrafish to 36°C heat shock has a significant effect on the growth of proximal and distal fin regenerates. Measurements were averaged from 18 untreated and 21 36°C-treated animals (*P<0.05, significantly different from no HS, t-test: **P<0.001, significantly different from no HS, t-test). (B) Graph of intrafin proximal to distal length ratios using animals described in A. The higher proximal:distal length ratio in 12 dpa 36°C heat-shocked animals indicates a greater sensitivity of distal regenerative growth to partial Fgfr inhibition (*P<0.001, significantly different from no HS, t-test). (C-E) Representative transgenics at 5 dpa given no HS (C) or 36°C heat shocks (D,E). Arrows indicate points of amputation. Some 36°C transgenics appeared similar to untreated transgenics at early timepoints such as 5 dpa (D), while others showed especially poor distal regeneration (E). Such variability is also reflected by the large standard error bars in B that characterize 36°C P:D ratios at 4 and 8 dpa. |
|
Positional memory is maintained in the absence of Fgf signaling. (A-C) Expression of mkp3 assessed by in situ hybridization in 3, 7 and 15 dpa single-amputated fins (33°C). The intensity of mkp3 expression signal wanes as regeneration progresses. (D) The PD length of the mkp3 signal is also reduced as regeneration progresses (n=10; *P<0.001, significantly different from 3 dpa, t-test; **P<0.005, significantly different from 3 and 7 dpa, t-test). (E-H) To test whether a long-term block of Fgf signaling could alter positional memory, 24 hsp70:dn-fgfr1 fins were amputated and exposed to 30 days of heat induction, followed by 15 days of room temperature treatment. Regeneration was fully blocked during the 30-day period. One of 24 animals showed no recovery after 15 days of restored Fgf signaling (E), while a small number (4/24) had regenerative blocks affecting more than half of the rays (F). The majority of regenerates displayed normal regeneration of more than half of rays (8/24, G) or all rays (11/24, H). EXPRESSION / LABELING:
|
|
A model for position-dependent regulation of regenerative growth rate. (A) After amputation, yet undetermined signals recognize position and establish cellular identity. These signals are thought to be present in a gradient along the PD axis, and introduce position-dependent properties of Fgf signaling. (B) The amount and PD length of Fgf signaling, each of which are greater in proximal regenerates, determine the PD length and the mitotic index of adjacent blastemal mesenchyme (blue). Because there is little overlap between highly proliferative blastemal tissue, regions of active Fgf signaling in the epidermis (green) and the distal region of the blastema (red), it is likely that an Fgf-dependent epidermal mitogen mediates blastemal proliferation (arrows). Greater influences by Fgf signaling on blastemal cells of proximal regenerates lead to higher growth rates than distal regenerates. |