- Title
-
Early Depletion of Primordial Germ Cells in Zebrafish Promotes Testis Formation
- Authors
- Tzung, K.W., Goto, R., Saju, J.M., Sreenivasan, R., Saito, T., Arai, K., Yamaha, E., Hossain, M.S., Calvert, M.E., Orbán, L.
- Source
- Full text @ Stem Cell Reports
|
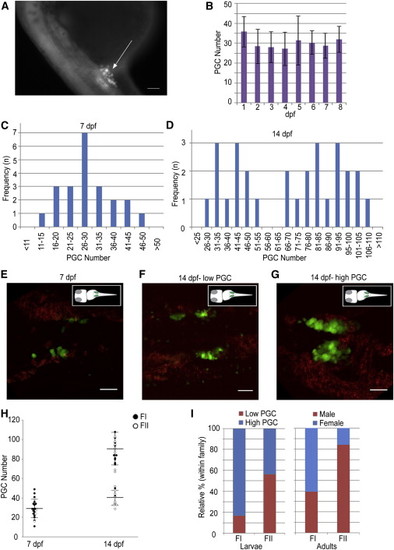
Dimorphic Proliferation of PGCs Happens during the Second Week in the Larval Gonads of the Zebrafish (A) A 24 hpf embryo of the Tg(vasa:vasa-EGFP) transgenic line. The GFP (+) germ cells cluster around anterior part of the yolk extension (arrow). The scale bar represents 100 µm. (B) The PGC number in the first 8 days of development counted using the squash method. Image and PGC counts obtained using a compound epifluorescence microscope (n = 10, 10, 12, 6, 5, 3, 4, and 3 at 1–8 dpf, respectively). The mean ± SD is shown. (C and D) Frequency distribution of PGC number at (C) 7 dpf (n = 22) and (D) 14 dpf (n = 28), showing unimodal and bimodal distributions, respectively. (E–G) Trunk regions of the Tg(vasa:vasa-EGFP) line, showing different PGC distributions at 7 and 14 dpf. Images are average intensity projections of confocal z stacks showing GFP (+) cells (green) and background autofluorescence (red). Scale bars represent 50 µm. (H) Density dot plot showing changes in the PGC number during development between different mating pairs (FI, closed circles; FII, open circles) at 7 and 14 dpf. Large horizontal bars indicate mean; smaller flanking bars indicate SD. (I) Relative percentages of individuals in low (<60, red) and high (>65, blue) PGC number groups within families (FI, n = 12; FII, n = 16) at 14 dpf (left) and the respective progeny sex ratios (male, red; female, blue; FI, n = 194; FII, n = 138). |
|
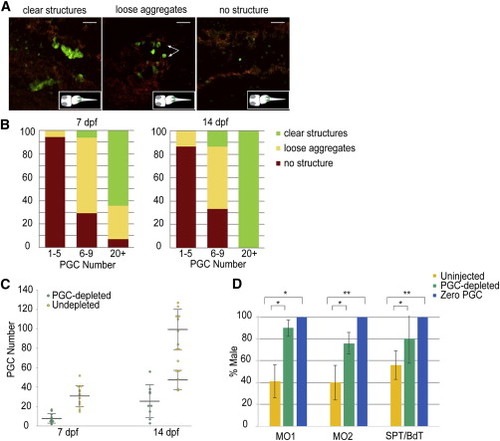
Masculinization Ensues in PGC-Depleted Zebrafish (A) Representative images of gonads show morphological variants following PGC depletion. Images are average intensity projections of confocal z stacks showing GFP (+) cells (green) and background autofluorescence (red). Scale bars represent 50 µm. (B) The relative percentage of morphological variants observed during development based on resultant PGC number at 7 and 14 dpf following depletion. (C) Density dot plot showing the stage-specific PGC number in PGC-depleted (6–9 PGCs, green; 7 dpf, n = 17 and 14 dpf, n = 15) and undepleted (>20 PGCs, yellow; 7 dpf, n = 14 and 14 dpf, n = 16) larvae. The resultant PGC number at 14 dpf in PGC-depleted larvae shows a unimodal distribution, while remaining bimodal in the undepleted ones. Large horizontal bars indicate mean; smaller flanking bars indicate the SD. (D) Relative percentage of male progeny resulting from two different methods of PGC-depletion: morpholino injection-MO1 (pairwise cross), MO2 (mass cross), and cell transplantation-SPT, BdT. The bar color indicates resultant PGC number zero (blue: n = 27, 29, 159 for MO1, MO2, and SPT/BdT), PGC-depleted (1–10 for MO1, 1–7 for MO2 and 1–9 for SPT/BdT, green; n = 111, 97, and 50 for MO1, MO2, and SPT/BdT) and uninjected controls (yellow: n = 237, 364, and 136 for MO1, MO2, and SPT/BdT). Bars indicate mean % ± SD of the mean percentages in four, six, four, and eight replicated experiments for MO1, MO2, SPT, and BdT, respectively. t test: p < 0.01; p < 0.001 calculated when PGC-depleted versus uninjected or zero PGC versus uninjected. See also Figures S1 and S2. |
|
Germline Chimeras Produced by Cell Transplantation, SPT and BdT, in Zebrafish (A) A single PGC with GFP fluorescence located at the gonadal ridge in a SPT chimera at the 25 somite stage. (B) The recipient of golden zebrafish (left) and the donor of Tg(vasa:DsRed2-vasa);Tg(bactin:EGFP) double transgenic embryos (right) used for the generation of BdT chimeras. (C) A donor blastoderm with GFP fluorescence attached to the host blastoderm a few hours after transplantation. (D) Donor-derived PGCs with RFP fluorescence were at the gonadal ridge at the prim-5 stage in a BdT chimera (arrow). (E–L) Representative female (E–G) and male (I–K) BdT chimeras were imaged under bright field (E and I), GFP fluorescence (F and J), and RFP fluorescence (G and K). Histological analysis of selected gonads (n = 5) from germline chimeras confirmed the presence of an ovary (H) or a testis (L). Scale bars represent 20 µm (A–D), 500 µm (E–G and I–K), and 100 µm (H and L). See also Table S1 for the survival rates following these manipulations and Movies S1 and S2 for BdT. |
|
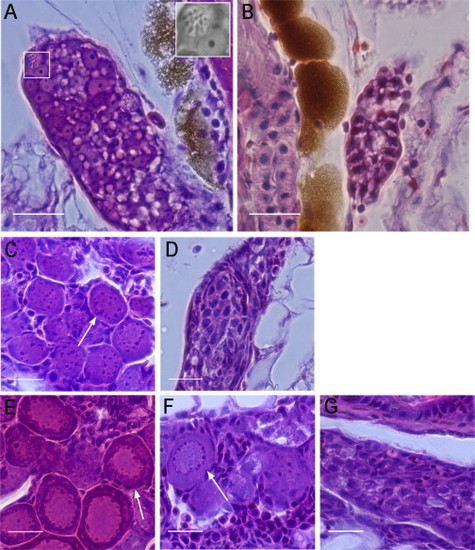
Hematoxylin and Eosin Staining of WT and PGC-Depleted Zebrafish Gonads at Different Developmental Stages (A) WT gonads at 15 dpf contained meiotic germ cells indicating oogonia (white box, inset). (B) PGC-depleted gonads at 15 dpf did not contain differentiated germ cells. (C and D) At 23 dpf, WT (C) and PGC-depleted gonads (D) are shown. Perinucleolar oocytes were only identified in the WT gonad (arrow). PGC-depleted gonads contained germ cells with one to several nucleoli. (E) WT gonads at 28 dpf showed slightly packed oocytes at the perinucleolar stage (arrow). (F and G) At 28 dpf, some PGC-depleted gonads have developed ovarian structures with perinucleolar oocytes (F, arrow), whereas others were similar histologically to PGC-depleted gonads of 23 dpf (compare G and D). Scale bars represent 20 µm. See also Figures S3 and S4. |