- Title
-
Wtip and Vangl2 are required for mitotic spindle orientation and cloaca morphogenesis
- Authors
- Bubenshchikova, E., Ichimura, K., Fukuyo, Y., Powell, R., Hsu, C., Morrical, S.O., Sedor, J.R., Sakai, T., and Obara, T.
- Source
- Full text @ Biol. Open
|
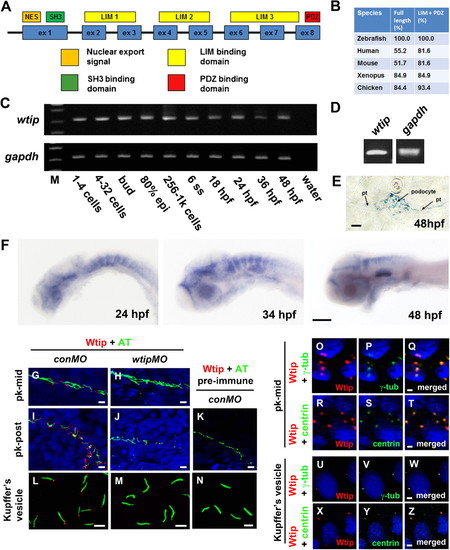
Zebrafish wtip gene and protein during development. (A) Zebrafish Wtip domain structure contains one nuclear export signal (NES, orange), one SH3 binding domain (SH3, green), three LIM domains (LIM1-3, yellow) and one PDZ binding domain (PDZ, red). (B) Percentage of amino acid identity between zebrafish Wtip and homologs of WTIP from other species. (C) RT-PCR time course of wtip expression during embryonic development. gapdh was used to control variations in total RNA (C,D). wtip expression was maternally deposited (C) and confirmed in the pronephros by RT-PCR using total RNA from isolated pronephros from 48 hpf embryos (D). (E) Cross-sections of the pronephric glomerulus and tubules. (F) Whole-mount in situ hybridization for wtip on embryos at 24 hpf, 34 hpf, and 48 hpf are side views anterior to the left and dorsal to the top. Zebrafish wtip expression is restricted to the eye, anterior brain, hindbrain, inner ear, and pronephros (E,F). Double immunofluorescence for the cilia marker acetylated α-tubulin (green) and Wtip (red) in confocal projections taken from whole-mount embryos for the middle segment of the pronephros at 24 hpf (G,H), the posterior pronephros at 24 hpf (I,J,K) and KV at the 10-somite stage (L,M,N). DAPI was used to counterstain nuclei (blue). Control embryos for antibody specificity using knockdown mediated by wtipMO of endogeneous Wtip protein translation (H,J,M) and with pre-immune serum (K,N) showed no expression. Localization of Wtip in the basal bodies of cilia was confirmed by double immunostaining with Wtip antibody (red) and either anti-γ-tubulin (P,V, green) or anti-centrin (S,Y, green), which are basal body markers in KV (V,Y, 10-somite stage) and the middle segment of the pronephros (P,S, 24 hpf). Pronephric tubules (pt), acetylated α-tubulin antibody (AT), γ-tubulin antibody (γ-tub), mid-pronephros (pk-mid), and posterior-pronephros (pk-post). Scale bars are 10 μm in E,G–N, 100 μm in F, and 2 μm in O–Z. |
|
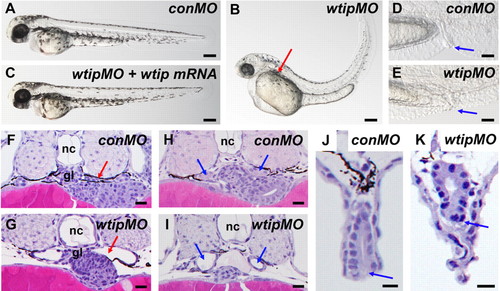
wtip knockdown embryos show pronephric cyst formation accompanied by cloaca malformation, hydrocephalus, body axis curvature and pericardial edema. (A,B,C) Side view of embryos at 48 hpf under light microscopy. (B) 48 hpf wtip morphants form pronephric cysts, hydrocephaly, body curvature and pericardial edema. The red arrow marks the location of the cyst dilation (B). (D,E) Lateral view of cloaca at 48 hpf. The blue arrow marks the cloaca. (C) wtip mRNA can rescue pronephric cyst, body curvature, hydrocephalus, and pericardial edema caused by wtipMO. Histological transverse-sections of 48 hpf embryos are 4µm JB-4 plastic sections stained with hematoxylin and eosin (F–K) at the level of the glomerulus (F,G; red arrow), anterior pronephros (H,I; blue arrow), and cloaca (J,K; blue arrow). Glomerular cysts (G), dilated anterior pronephros (I) and cloaca malformation (K) were observed in the 48 hpf wtip morphants. Control morpholino injected embryos (conMO), wtip morpholino injected embryos (wtipMO), glomerulus (gl), and notochord (nc). Scale bars are 200 μm in A–C, 500 μm in D,E, and 100 μ in F–K. PHENOTYPE:
|
|
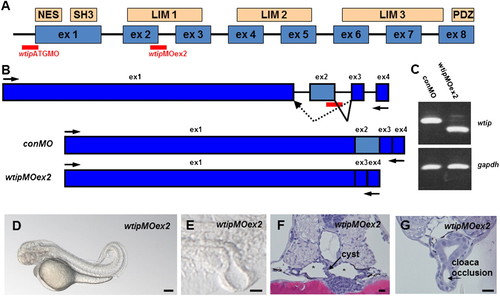
Similar to wtip knockdown, wtipMOex2 morphants resulted in pronephric cyst formation accompanied by cloaca malformation. (A) Predicted domain structure of zebrafish Wtip protein. The putative nuclear export sequence (NES), SH3 binding domains, three LIM domains and the PDZ binding domain (PDZ) are shown in boxes. Anti-sense morpholino to target the translational initiation site of wtip mRNA (wtipMO) was used for wtip knockdown and wtip exon2 specific MO (wtipMOex2) was used to target the exon2/intron2 junction. (B) To test the function of wtip in zebrafish, we targeted splice donor sites in wtip exon2 with MO. The extent to which steric blockade of mRNA splicing caused alterations in wtip mRNA processing was quantified by RT-PCR using flanking exon primers. Injection of wtipMOex2 at the one- to two-cell stage resulted in an in-frame deletion of exon 2 and resulted in the joining of exon3 to exon1, thus deleting most of the first LIM domain. (C) The efficacy of the injected wtipMOex2 was quantified at 48 hpf by RT-PCR. The upper panel shows the result of RT-PCR with primers in exons1 and 4 of wtip. The lower panel shows the result of RT-PCR with primers for gapdh, used to control RNA amounts. (D) Side view of 48 hpf wtipMOex2 morphants displaying pronephric cysts, hydrocephalus, body curvature and pericardial edema. (E) Lateral view of cloaca at 48 hpf shows cloaca malformation. Histological transverse-sections of 48 hpf wtipMOex2 morphant embryos are 4μm JB-4 plastic sections stained with hematoxylin and eosin (F,G) at the level of the glomerulus cysts (F, black arrow), tubular cysts (F, asterisks) and cloaca (G, black arrow). Scale bars are 200 μm in D and 10 μm in E–G. PHENOTYPE:
|
|
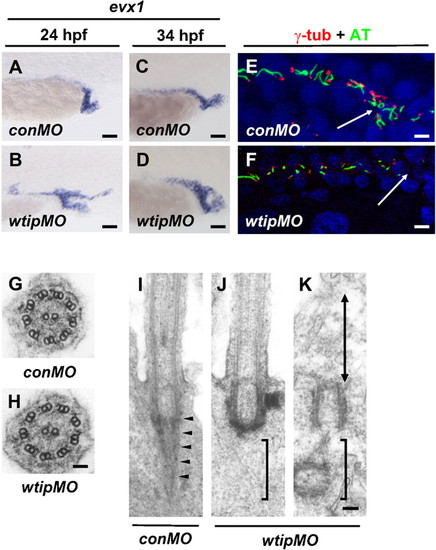
In wtip knockdown embryos cloaca malformation is associated with fewer ciliated cells and the lack of rootlet structures, which may compromise the motility of cilia. Lateral views of evx1 expression in control (A,C) and wtip morphants (B,D) in the posterior pronephros and cloaca at 24hpf (A,B) and 34 hpf (C,D). In 24 hpf (A) and 34 hpf (C) control embryos, evx1 was expressed in the epidermis and cloaca. In wtip morphants, evx1 was expressed broadly around the area of the posterior pronephros and cloaca at 24 hpf (B). evx1 continued to be expressed at 34 hpf in the malformed cloaca (D). Confocal projections of a double immunofluorescence of the posterior pronephros and cloaca in 24 hpf control (E) and wtip morphant (F) embryos labeled with anti-γ-tubulin (basal body, red) and anti-acetylated α-tubulin (cilia, green) revealed fewer ciliated cell in the wtip morphants (F). The white arrow points to the cloaca area. Transmission electron micrographs of the transverse sections in 34 hpf control embryos (G) and wtip morphants (H) revealed an axoneme with 9+2 structure. Longitudinal sections in control embryos displayed a striated rootlet structure (arrowheads) located adjacent to the basal body (I). In wtip morphants, ciliated cells exhibited a basal body but lacked rootlet structure (J), and non-ciliated (double arrow) cells exhibited mother centrioles/basal bodies, which were separated from the inner leaflet of the apical cell membrane (K). Scale bars are 25 μm in A–D, 100 μm in E,F, 50 nm in G,H, and 100 nm in I–K. |
|
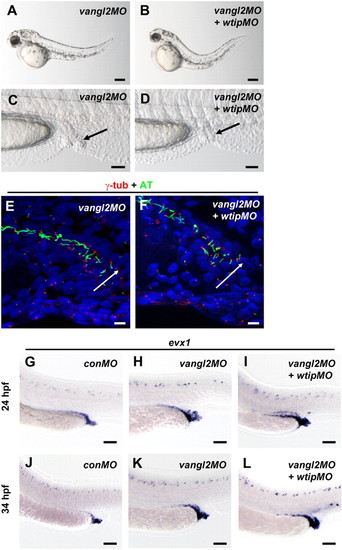
In vangl2 knockdown embryos, cloaca malformation is associated with fewer ciliated cells. (A,B) Side view of embryos at 48 hpf imaged with light microscopy. (C,D) Lateral view of cloaca at 48 hpf. The black arrow marks the cloaca. 48 hpf vangl2 (A,C) and vangl2 + wtip (B,D) morphants form pronephric cysts, cloaca malformation, hydrocephalus, body curvature and pericardial edema. Confocal projections of a double immunofluorescence of the posterior pronephros and cloaca in 24 hpf vangl2 (E) and vangl2 + wtip morphant (F) embryos labeled with anti-γ-tubulin (basal bodies, red) and anti-acetylated α-tubulin (cilia, green) revealed fewer ciliated cells in the vangl2 (E) and vangl2 + wtip morphants (F). The white arrow points to the cloaca area. Lateral views of evx1 expression in control (G,J), vangl2 (H,K) and vangl2 + wtip morphants (I,L) in the posterior pronephros and cloaca at 24hpf (G–I) and 34 hpf (J–L). In 24 hpf (G) and 34 hpf (J) control embryos, evx1 was expressed in the epidermis and cloaca. In vangl2, and vangl2 + wtip morphants evx1 was expressed broadly around the area of the posterior pronephros and cloaca at 24 hpf (H,I). evx1 continued to be expressed at 34 hpf in the malformed cloaca (K,L). Scale bars are 200 μm in A,B, 50 μm in C,D,G–L, and 10 μm in E,F. |
|
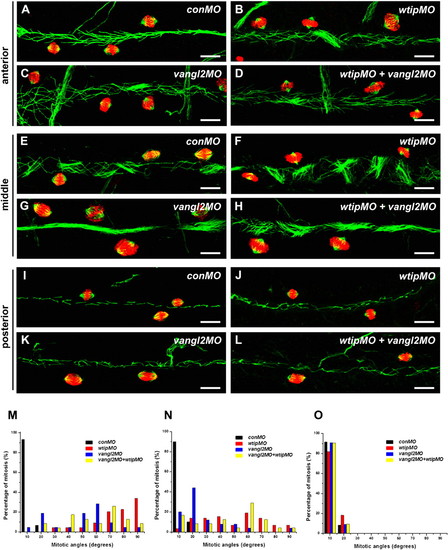
wtip knockdown leads to mitotic cell division defects in the pronephros. Whole-mount immunofluorescence of mitotic spindles for the 48 hpf pronephros were stained with anti-acetylated α-tubulin (cilia, green), anti-α-tubulin (spindle, green) and anti-phosphorylated histone H3 (pH3, red) for the anterior (A–D), middle (E–H) and posterior (I–L) pronephros. In control embryos, cells with mitotic spindle fibers oriented in the longitudinal plane of the anterior (A), middle (E) and posterior (I) pronephros. In the anterior (A–D) and middle (E–H) pronephros, wtip morphants (B,F,J), vangl2 morphants (C,G,K) and wtip + vangl2 morphants (D,H,L) resulted in mitotic spindle orientation defects. The posterior pronephros (I–K) showed mitotic spindles oriented normally in the longitudinal plan (I–L). Mitotic spindle angles were photographed by confocal imaging in the three segments of the anterior (A–D,M), middle (E–H,N) and posterior (I–L,O) pronephros at 48 hpf. The angle between the long axis of the pronephros and the spindle fibers was measured; angles were grouped into 10 degree bins. Scale bars are 100 μm in A–L. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|