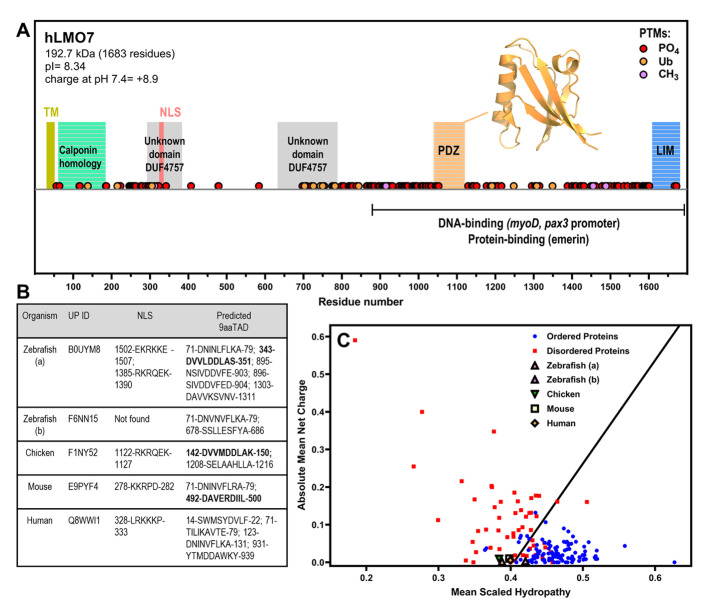
Figure 3 LMO7 is a multidomain protein that has a charge–hydropathy characteristic of proteins enriched in intrinsic disorder and contains nuclear localization sequence(s) and putative nine-amino-acid transactivation motifs. (A) Schematic organization of human LMO7 with the domains, motifs and post-translational modifications (PTMs) indicated along sequence. The C-terminal domain mediates interaction with DNA promoters and protein partners. TM, transmembrane α-helix. NLS, nuclear localization sequence. PO4, phosphorylation. Ub, ubiquitination. CH3, methylation. The inset shows the X-ray crystallographic structure of the PDZ domain (PDB 2eaq). (B) LMO7 from different species were analyzed by NLSdb (third column, https://rostlab.org/services/nlsdb/, 20 November 2021) and 9aaTAD (fourth column, https://www.med.muni.cz/9aaTAD/). Perfect matches of 9aaTAD are highlighted in bold whereas other annotated motifs correspond to 92% matches. Corresponding UniProt identifiers (UP ID) are shown in the second column. (C) Predictor of Natural Disordered Regions (PONDR; http://www.pondr.com/) analysis of charge–hydropathy (CH plot). Well-folded proteins indicated as blue circles and disordered proteins as red squares. LMO7 from the different species is located in the plot region of disordered proteins apart from isoform b of zebrafish.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Int. J. Mol. Sci.