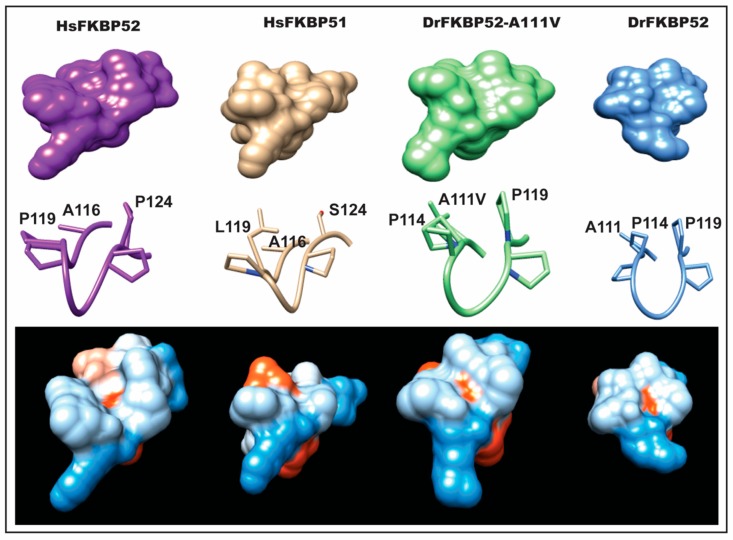
Figure 6
Predicted structural differences in the FK1 proline-rich loop. Homology modeling was used to generate predicted models of DrFKBP52 and DrFKBP52-A111V, in the comparison to human FKBP52 and FKBP51 to determine conformational changes induced by the DrFKBP52-A111V mutation. Crystal structures of HsFKBP51 (PDB ID: 1KT0), HsFKBP52 (PDB ID: 1Q1C), DrFKBP52 (predicted), and DrFKBP52-A111V (predicted) are aligned; the respective FK1 domains are shown in space-filled modeling and colored in hydrophobicity scale (blue = hydrophilic, red = hydrophobic). Note that the only differences between FKBP51 and HsFKBP52 within the loop region are at positions 119 and 124.