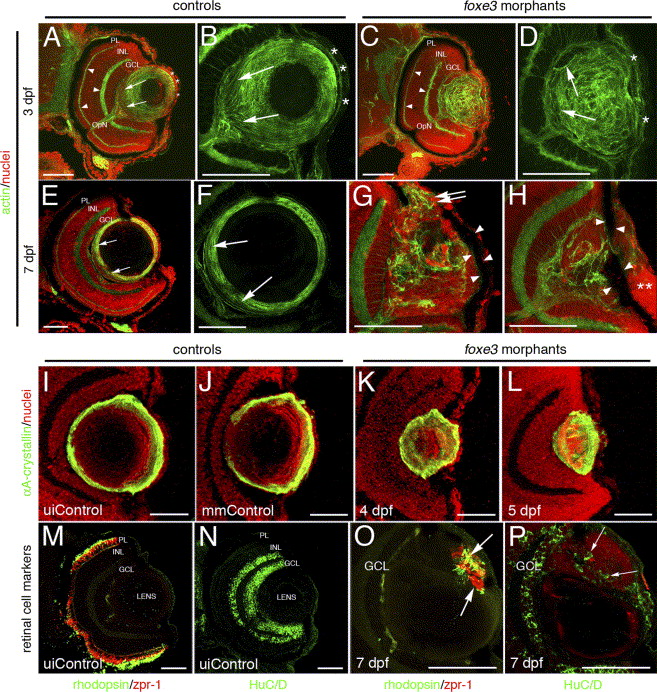
Fig. 9 Immunohistochemical analysis of foxe3 morphant lens and retina. Frozen eye sections of uninjected controls (panels A, B, E and F) and foxe3 morphants (panels C, D, G and H) were stained with phalloidin-AF488 and propidium iodide to label actin filaments and cell nuclei, respectively. At 3 dpf in the uninjected control, actin is localized in the retinal plexiform layers (panel A, arrowheads) and the membranes of the lens cells (panel A, low magnification; panel B, high magnification). The phalloidin-stained 3 dpf control lens (panel B) demonstrates actin localization in the elongating lens fibers near the proximal lens pole (arrows) and epithelial cell membranes (asterisks). The 3 dpf morphant retinal structure is equivalent to the control based on the actin staining pattern (compare panel C to A, arrowheads), while the morphant lens actin localization pattern is abnormal (panels C and D). At 3 dpf, the morphant lens (panel D) exhibits a failure of lens fiber elongation (arrows) and disorder in the fiber cell and epithelial cell (asterisks) populations. At 7 dpf, the control lens actin staining (panels E and F) is restricted to the cortical fibers (green) and overlaps with the more peripheral population of nucleated cells (panel E, low magnification; panel F, high magnification shows only the actin staining). The region of elongating fiber cells can be identified (panels E and F, arrows). Two different 7 dpf morphant lens are shown in panels G and H, with both revealing many nucleated cells (red) that fail to express actin (green). Nucleated cells are contiguous between the morphant lens and the cornea (panel G, double arrows). In addition, pigmented cells cover the lens (panels G and H, arrowheads) and the corneal nuclear arrangement is abnormal (panel H, asterisks). αA-crystallin protein was immunolocalized in the lens of control and foxe3 morphants (panels I–L). In the uninjected and mismatch control morpholino-injected embryos (panels I and J, respectively) at 5 dpf, high levels of αA-crystallin protein (green signal) are detected in the lens periphery. αA-crystallin is also detected in the morphant lens at 4 and 5 dpf (panels K and L, respectively), although the localization pattern differs from the controls (compare panels K and L to I and J). Retinal cell antigens were also immunolocalized in control and foxe3 morphant frozen sections (panels M–P). In the controls, rhodopsin and zpr-1 antibodies label the rod and double cone photoreceptors, respectively (panel M, green and red signals, respectively), while HuC/D protein is detected in differentiated amacrine and ganglion cells (panel N, green signal). Some morphant lens display aberrant expression of retinal cell markers. The morphant lens in panel O exhibits rhodopsin and zpr-1 expression (arrows), while the morphant lens in panel P displays abberant HuC/D expression (arrows). The green signal on the posterior surface of the lens in panel O represents autofluorescence and not primary antibody detection. Scale bars = 50 μm. Abbreviations: PL, photoreceptor layer; INL, inner nuclear layer; GCL, ganglion cell layer; OpN, optic nerve; uiControl, uninjected control and mmControl, mismatch morpholino-injected control.
Reprinted from Mechanisms of Development, 123(10), Shi, X., Luo, Y., Howley, S., Dzialo, A., Foley, S., Hyde, D.R., and Vihtelic, T.S., Zebrafish foxe3: Roles in ocular lens morphogenesis through interaction with pitx3, 761-782, Copyright (2006) with permission from Elsevier. Full text @ Mech. Dev.