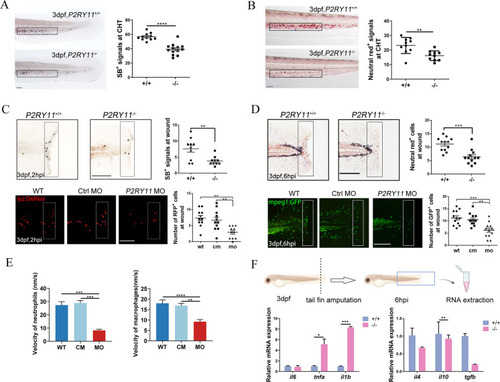
Deficiency of P2RY11 reduced macrophages and neutrophils and their accumulation at the injury site following the fin amputation. A, The Sudan black B (SB) signal in siblings (upper) and P2RY11 mutants (lower) at 3 dpf (t-test, n = 10). B, The neutral red signal in siblings (upper) and P2RY11 mutants (lower) at 3 dpf in CHT (t-test, n = 10). C, recruited neutrophils to wounds following tail fin amputation. SB staining (upper) showed the recruitment of neutrophils at 2 hpi in 3dpf-siblings and P2RY11 mutants (t-test, n = 10). SB+ cells were significantly reduced in P2RY11−/− mutants in the quantified region (the black rectangle). The recruitment of neutrophils (RPF+ cells in the white rectangle) was also abolished in P2RY11 MO morphants at 3 dpf in the transgenic lyz:DsRed compared with the control morpholino-injected group (cm) and wild type group (WT) (one-way ANOVA, n = 11). D, recruited macrophages to wounds following tail fin amputation. Neutral red staining revealed the recruitment of macrophages at 6 hpi in 3dpf-siblings and P2RY11 mutants (t-test, n = 12). Neutral red+ cells were significantly reduced in P2RY11 mutants within the quantified region (the black rectangle). The recruitment of macrophages (GFP+ cells in the white rectangle) was also abolished in P2RY11 MO morphants at 3 dpf in the transgenic mpeg1: GFP compared with the control morpholino-injected group (CM) and wild- type group (WT) (one-way ANOVA, n = 12). E, Migration speed of neutrophils and macrophages in P2RY11 knock-down larvae during the process toward the wound edge (one-way ANOVA, n = 5). F, Quantitative analysis of il6, tnfa, il1b, il4, il10, and tgfb from tails of WT and mutants at 6 hpi (t-test, n = 20). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Scale bar: 200 μm
|

