Fig. 1
- ID
- ZDB-FIG-230925-20
- Publication
- Yumimoto et al., 2023 - Molecular evolution of Keap1 was essential for adaptation of vertebrates to terrestrial life
- Other Figures
- All Figure Page
- Back to All Figure Page
|
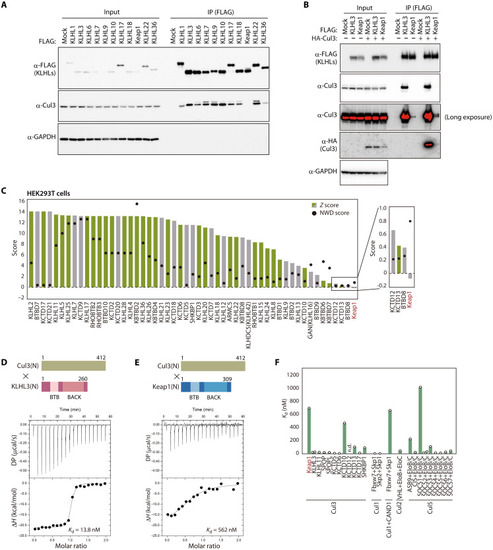
Weaker interaction of Keap1 with Cul3 compared with other receptors.
(A) Lysates of HEK293T cells expressing FLAG-tagged human KLHL proteins were subjected to immunoprecipitation (IP) with antibodies to FLAG, and the resulting precipitates as well as the original lysates (Input) were subjected to immunoblot analysis with the indicated antibodies (α-). GAPDH was examined as a loading control. (B) Lysates of HEK293T cells expressing FLAG-tagged human KLHL3 or Keap1 together with HA-tagged human Cul3 were subjected to immunoprecipitation with antibodies to FLAG, and the resulting precipitates as well as the original lysates were subjected to immunoblot analysis with the indicated antibodies. GAPDH was examined as a loading control. (C) Fifty-three BTB proteins previously found to interact with Cul3 in HEK293T cells (25). Z and NWD scores were obtained from CompPASS analysis (26). Cul3 interactants in the BioPlex 3.0 database (27) are shown in green, with others shown in gray. (D and E) Representative ITC titration curves for interaction between human Cul3(N) and either human KLHL3(N) (D) or human Keap1(N) (E) as well as domain organization of the protein fragments. Baseline-corrected differential power (DP) versus time as well as the normalized binding curve, with integrated changes in enthalpy (ΔH) plotted against molar ratio, are shown. Each experiment was performed independently at least twice. (F) Dissociation constants for Cullins and the indicated receptors. Values other than those for Keap1 and KLHL3 were obtained from previous studies (21, 23, 28, 54–62). n.d., not detected. |