Fig. 3
- ID
- ZDB-FIG-230225-24
- Publication
- Wu et al., 2021 - Histone H2A Nuclear/Cytoplasmic Trafficking Is Essential for Negative Regulation of Antiviral Immune Response and Lysosomal Degradation of TBK1 and IRF3
- Other Figures
- All Figure Page
- Back to All Figure Page
|
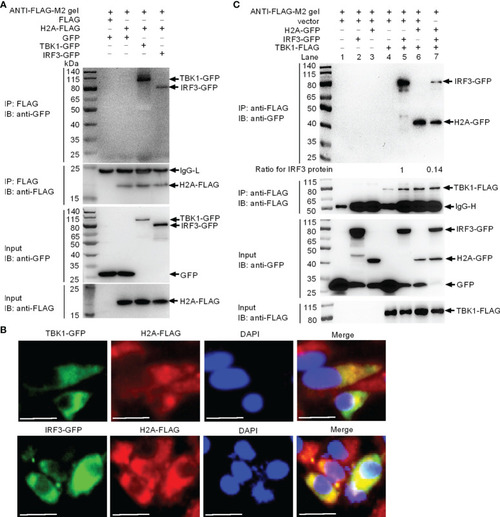
Zebrafish histone H2A interacts with TBK1 and IRF3 to disrupt the formation of TBK1-IRF3 complex. (A) Zebrafish histone H2A interacted with TBK1 and IRF3. (B) Zebrafish histone H2A colocalized with TBK1 and IRF3. (C) Zebrafish histone H2A interacted with TBK1 to inhibit the formation of TBK1-IRF3 complex. For (A, C), co-IP was performed with anti-FLAG-conjugated agarose beads in EPC cells. The cell lysates and bound proteins were analyzed by immunoblotting with the indicated Abs. All experiments were repeated for at least three times with similar results. The expression ratio for IRF3 protein was quantified by Quantity One. For (B), immunofluorescence analysis was performed in EPC cells transfected with H2A-FLAG and TBK1-GFP or IRF3-GFP. Scale bar: 25 μm. |