FIGURE
Fig. 1
- ID
- ZDB-FIG-220706-74
- Publication
- Bielak et al., 2022 - N'-terminal- and Ca2+-induced stabilization of high-order oligomers of full-length Danio rerio and Homo sapiens otolin-1
- Other Figures
- All Figure Page
- Back to All Figure Page
Fig. 1
|
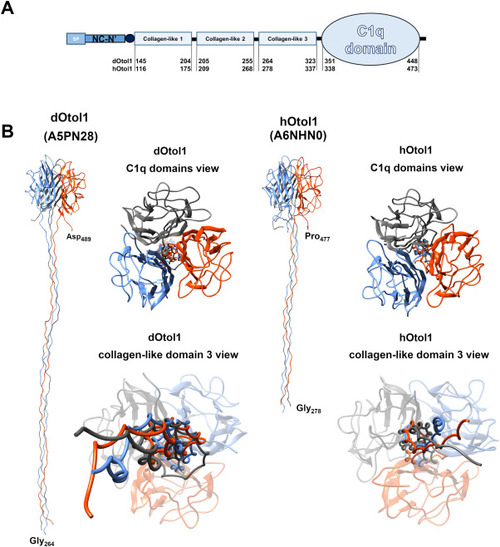
Fig. 1. Schematic representation of the domain-specific structure of otolin-1 based on UniProt data for otolin-1 with identifiers A5PN28 and A6NHN0 (A). Given segments (SP – signal peptide; NC-N′ – noncollagenous N-terminal domain; central collagen-like domain and C-terminal globular C1q domain) are captioned with amino acid positions in the full primary sequence of the protein. Panel B shows fragments of otolin-1 trimeric structure models composed of the collagen-like domain 3 and C1q domains. |
Expression Data
Expression Detail
Antibody Labeling
Phenotype Data
Phenotype Detail
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Reprinted from International journal of biological macromolecules, 209(Pt A), Bielak, K., Hołubowicz, R., Zoglowek, A., Żak, A., Kędzierski, P., Ożyhar, A., Dobryszycki, P., N'-terminal- and Ca2+-induced stabilization of high-order oligomers of full-length Danio rerio and Homo sapiens otolin-1, 1032-1047, Copyright (2022) with permission from Elsevier. Full text @ Int. J. Biol. Macromol.