Fig. 4.
- ID
- ZDB-FIG-220205-48
- Publication
- Dal et al., 2021 - The zebrafish embryo as an in vivo model for screening nanoparticle-formulated lipophilic anti-tuberculosis compounds
- Other Figures
- All Figure Page
- Back to All Figure Page
|
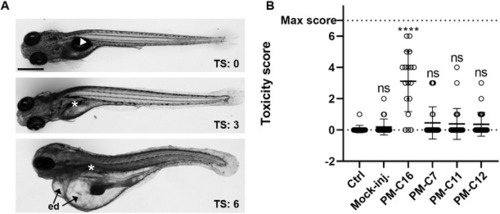
Evaluation of the in vivo toxicity of PM-formulated nNF derivatives in zebrafish embryos by toxicity score. Zebrafish embryos were injected with high doses (50 mg/kg) of PM-formulated C7, C11 and C12, or 5 mg/kg of PM-C16, and the toxicity score (TS) was determined at 4 dpt in individual embryos based on morphological and physiological indicators of toxicity, as specified in Table 1. (A) Representative images of embryos with different toxicity scores displaying normally inflated (arrowhead) or lack of inflated swim bladder (*), severe edemas (ed) and abnormally curved body shape (bottom image). Scale bar: 500 μm. (B) Toxicity scores in embryos injected with PM-formulated compounds. Non-injected (ctrl) and mock (PBS)-injected embryos were used as negative toxicity controls, and embryos injected with PM-C16 as a positive toxicity control. Each symbol represents the toxicity score of an individual larvae and the mean for each group is shown as a horizontal line with error bars denoting the s.d. Ctrl, n=18; Mock-inj., n=21; C16, n=18; C7, n=31; C11, n=26; C12, n=25); ***P<0.001 compared with the non-injected control (ctrl); ns, not significant (non-parametric Kruskal–Wallis H test followed by post-hoc analysis using Dunn's multiple comparisons test). |