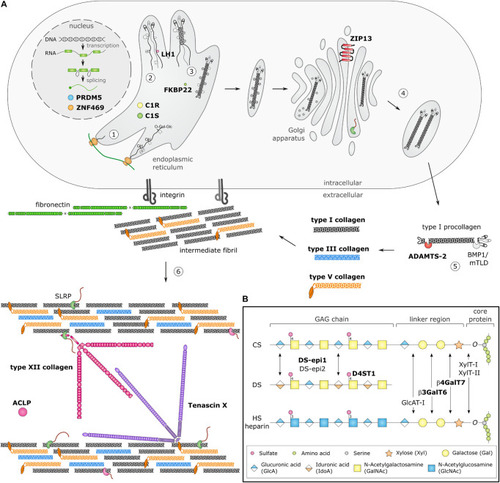
Schematic overview of collagen and glycosaminoglycan (GAG) biosynthesis and collagen fibrillogenesis. Molecules defective in Ehlers-Danlos syndromes (EDS) are highlighted in bold. (A) Fibrillar collagen biosynthesis starts with transcription and translation of pro-α-chains (step 1). Nascent pro-α-chains are heavily post-translationally modified by several proline and lysine hydroxylases and galactosyltransferases (step 2). The association of the C-terminal propeptides of three pro-α-chains, initiates triple helix formation which propagates to the N-terminus in a zipperlike fashion and is assisted by several molecular chaperones (step 3). The trimeric procollagen molecules aggregate laterally, are transported in secretory vesicles and are eventually directed to the extracellular environment (step 4). Removal of the N- and C-propeptides, by ADAMTS-2 and BMP-1/mTLD, respectively, results in the formation of a collagen molecule (step 5) that can then assemble into highly ordered striated fibrils. The tissue-specific assembly of collagen fibrils requires the concerted action of several assisting proteins, categorized as organizers, nucleators and regulators (step 6). At the plasma membrane, fibronectin and integrins serve as organizers of fibril assembly. Some collagens, such as type V collagen, function as nucleators, which initiate immature fibril assembly at the cell surface. Type V collagen co-assembles with type I collagen into heterotypic fibrils with the entire triple helical domain of type V collagen embedded within the fibril, whereas its partially processed N-propeptide domain protrudes to the fibril surface and controls fibrillogenesis by sterically hindering the addition of collagen monomers. The intermediate fibrils are then deposited into the extracellular matrix (ECM). Stabilization of these fibrils is provided by interactions with regulators such as the small leucine-rich proteoglycan (SLRP) decorin, tenascin-X and type XII collagen, which influence the rate of assembly, size and structure of the collagen fibrils. As fibrillogenesis proceeds, fibril growth occurs through linear and lateral fusion of intermediate collagen fibrils which are subsequently stabilized by the formation of covalent intra- and inter-molecular cross-links. (B) GAG biosynthesis starts with the synthesis of a proteoglycan core protein which is subsequently modified by several Golgi-resident enzymes. Initially, a common linker region containing four monosaccharides is formed. Biosynthesis of this tetrasaccharide linker region starts with the stepwise addition of a xylose (Xyl) residue to a specific serine residue of the core protein, catalyzed by xylosyltransferase-I and II (XylT-I/-II). Subsequently, two galactose (Gal) residues are added by galactosyltransferase-I (GalT-I or β4GalT7) and galactosyltransferase-II (GalT-II or β3GalT6). Finally, the addition of a glucuronic acid (GlcA), catalyzed by glucuronosyltransferase-I (GlcAT-I) completes the formation of the linker region. The alternating addition of either N-acetyl-glucosamine (GlcNAc) or N-galactosyl-glucosamine (GalNAc) and GlcA defines the composition of the GAG-chain and subdivides proteoglycans into heparan sulfate (HS) proteoglycans and chondroitin sulfate (CS)/dermatan sulfate (DS) proteoglycans. The GAG-chains are then further modified by epimerization and sulfation. DS synthesis necessitates the epimerization of GlcA towards iduronic acid (IdoA), which is catalyzed by DS epimerases–1 and -2 (DS-epi1 and DS-epi2). Subsequently, dermatan 4-O-sulfotransferase-1 (D4ST1) is able to catalyze 4-O-sulfation of GalNAc, thereby preventing back-epimerization of the adjacent IdoA.
|

