|
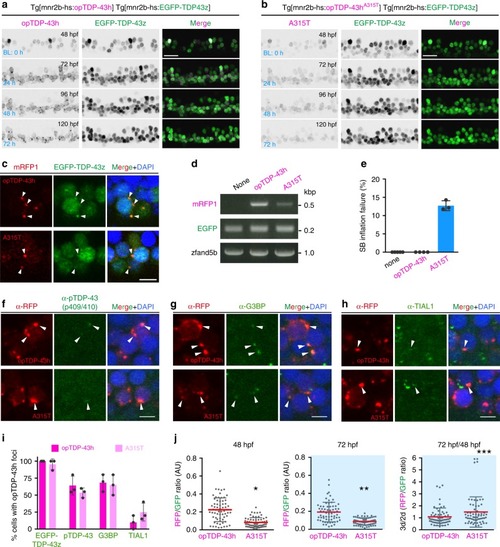
The IDR mutation A315T enhances protein stability and oligomerization-dependent toxicity.a, b Live imaging of the spinal motor column from 48 to 120 hpf. c Chronically light-stimulated opTDP-43h (top) and opTDP-43hA315T (bottom) aggregate in the cytoplasm and seed EGFP-TDP-43z aggregation. Arrowheads indicate opTDP-43 and opTDP-43hA315T aggregates that contain EGFP-TDP-43z. d RT-PCR analysis for opTDP-43h and opTDP-43hA315T transcripts at 72 hpf. e Failure rate of swimming bladder (SB) inflation of Tg[mnr2b-hs:EGFP-TDP43z] (none), Tg[mnr2b-hs:EGFP-TDP43z] Tg[mnr2b-hs:opTDP-43h], and Tg[mnr2b-hs:EGFP-TDP43z] Tg[mnr2b-hs:opTDP-43hA315T] (A315T) larvae at 120–144 hpf. The average failure rates were defined from at least three independent assays where six or more fish were illuminated (Source data are provided as a Source Data file). SB inflation failure was not observed when fish were raised under normal dark light cycles (N > 100 for each). f–i Immunofluorescence analyses of phospo-TDP-43 (f), G3BP (g), TIAL1 (h) against cytoplasmic opTDP-43h and opTDP-43hA315T aggregates at 120 hpf. At least, twenty cells with distinct opTDP-43h or opTDP-43hA315T aggregates were examined for each of three independent fish. j Stability of opTDP-43h or opTDP-43hA315T. Fluorescence intensities of opTDP-43h or opTDP-43hA315T (RFP) relative to EGFP-TDP-43z (GFP) were examined at 48 hpf and 72 hpf, in the same set of 64 cells from three independent animals. *p < 0.0001, **p < 0.0001, ***p = 0.03 (unpaired t-test, two-tailed). The bars indicate 20 µm (a, b) and 5 µm (c, f–h). Error bars show SD.
|