Fig 8
- ID
- ZDB-FIG-200229-26
- Publication
- Concilio et al., 2020 - Inter-species variation in monovalent anion substrate selectivity and inhibitor sensitivity in the sodium iodide symporter (NIS)
- Other Figures
- All Figure Page
- Back to All Figure Page
|
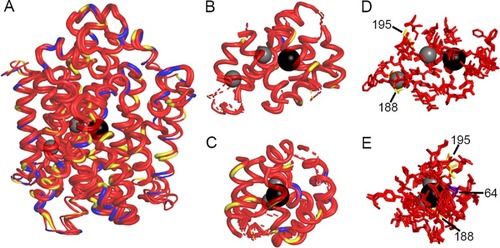
(A) Overlap of hNIS, wNIS, and zNIS. The thickness of the alpha-helices and loops represent per-residue RMSD values calculated between the corresponding residues of hNIS, wNIS, and zNIS with hNIS as a reference. Thick alpha helices and loops signify low RMSD values (regions with high structural similarity). Color coding: red–fully conserved residues between hNIS, wNIS, and zNIS; yellow–substitution with a chemically similar residue in wNIS and zNIS; blue–substitution with non-similar residue; grey spheres–Na+ ions, black sphere–I- ion. (B-C) Two different projections of protein areas within 10Å of Na+ and within 15Å of I-. Dashed lines indicate some or all of residues in the alpha helix are outside the 10/15Å cutoff. (D-E) Two different projections of residues from the ion coordination spheres (within 5Å) identified from MD simulations. The residue numbers of the non-conserved residues are also shown. |