Fig. 10
|
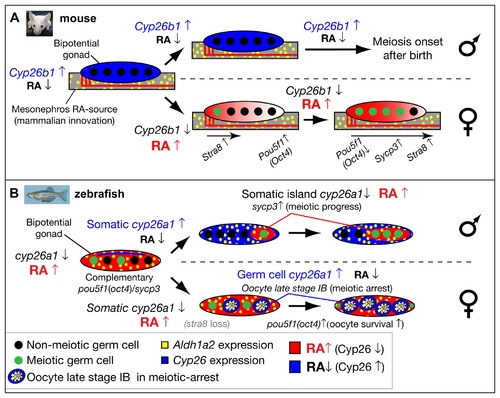
Model for the role of retinoic acid and meiotic progression during gonadogenesis in mouse and zebrafish. In mouse (A), Aldh1a2 (yellow) in the mesonephros provides the RA-source that regulates gonad development (reviewed in [18]), while in zebrafish (B), aldh1a2 is expressed by somatic cells within the gonad (yellow), and thereby provides an internal RA-source. In mouse, non-meiotic germ cells (black circles) in bipotential gonads are protected from RA by high expression of Cyp26b1 (blue), while in zebrafish, Cyp26a1 expression (blue) is restricted to cells at the dorsal surface near the body cavity, and thereby germ cells elsewhere in the gonad are not protected from RA (red) and they are able to enter into meiosis (green circles). In mouse, sexually dimorphic expression of Cyp26b1 causes low levels of RA (blue) in testes (males, top in A) and high levels of RA (red) that diffuses from the mesonephros to the ovaries in an anterior to posterior wave (females, bottom in A), which results in a sexually dimorphic onset of meiosis. The onset of meiosis in mouse follows an anterior–posterior wave, accompanied by up-regulation of Stra8, and Sycp3 and down-regulation of Pou5f1(Oct4) [18]. In zebrafish, the sexually dimorphic expression of cyp26a1 differs in time and location from those of Cyp26b1 in mouse. In zebrafish males (top in B), somatic cells up-regulate cyp26a1, and meiotic cells expressing sycp3 localize to somatic islands (red) that lack cyp26a1 expression. This model is consistent with a role for Cyp26a1 in degrading RA and thereby protecting nearby germ cells from progressing through meiosis. Consistent with this model, in females (bottom in B), cyp26a1 is not expressed in somatic cells, but it is up-regulated in the ooplasm (blue) of oocytes at late stage IB that have reached diplotene stage and have entered in meiotic arrest, and also express pou5f1(oct4). The co-expression of cyp26a1 and pou5f1(oct4) is compatible with the proposed roles of these genes in mouse on promoting germ cell survival and preventing apoptosis [41, 86], mechanisms that have been shown to be central for tipping the sexual fate of the gonad toward the female pathway in zebrafish [ 5, 8, 87]. |