Fig. 1
- ID
- ZDB-FIG-120601-14
- Publication
- Chablais et al., 2012 - The regenerative capacity of the zebrafish heart is dependent on TGFβ signaling
- Other Figures
- All Figure Page
- Back to All Figure Page
|
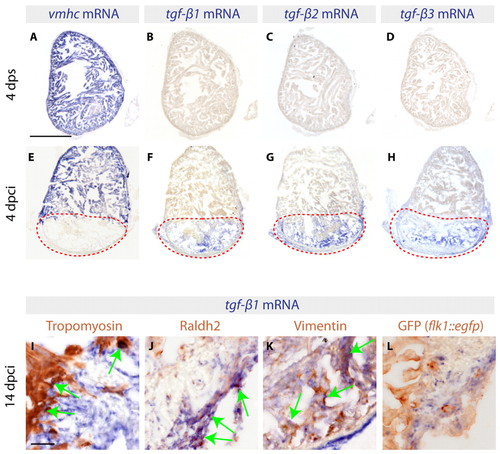
Three TGFβ ligands are expressed in the infarct zone following cryoinjury. (A-H) In situ hybridization of consecutive heart sections with vmhc, tgfb1, tgfb2 and tgfb3 mRNA antisense probes (blue) of control zebrafish hearts at 4 days post sham surgery (dps) (A-D) and of cryoinjured hearts at 4 days post cryoinjury (dpci) (E-H). Red dashed lines encircle the injured areas. (A,E) At 4 dps, vmhc expression labels the intact ventricle. (B-D) None of the three TGFβ transcripts is detected in uninjured hearts. (E) The absence of vmhc mRNA demarcates the infarct area at 4 dpci. (F-H) At 4 dpci, tgfb1, tgfb2 and tgfb3 are upregulated in the injured area. (I-L) Heart sections at 14 dpci double-stained by in situ hybridization using tgfb1 probe (blue) and immunohistochemistry using different cell type markers (brown). Arrows indicate double-positive cells. (I) Intact Tropomyosin-labeled myocardium does not express tgfb1, with the exception of cardiomyocytes abutting the infarct. (J) Raldh2-positive epicardial cells invading the injury area are co-labeled by tgfb1 staining. (K) Vimentin-expressing fibroblasts of the infarct express tgfb1. (L) GFP expressed under the endothelial cell promoter flk1 (kdrl – Zebrafish Information Network) does not colocalize with tgfb1. Scale bars: 300 μm in A; 50 μm in I. |