Fig. 8
- ID
- ZDB-FIG-100429-85
- Publication
- van Eekelen et al., 2010 - RPTPalpha and PTPepsilon Signaling via Fyn/Yes and RhoA is Essential for Zebrafish Convergence and Extension Cell Movements during Gastrulation
- Other Figures
- All Figure Page
- Back to All Figure Page
|
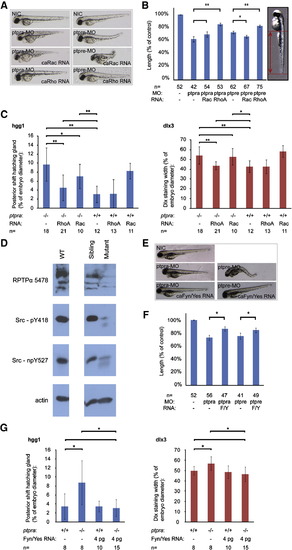
RPTPα and PTPε knockdown rescued by active RhoA or active Fyn and Yes. (A) Zebrafish embryos were micro-injected at the 1-cell stage with ptpra-MO (0.3 ng/embryo) or ptpre-MO (2.5 ng ptprea-MO + 2.5 ng ptpreb-MO/embryo) alone or co-injected with active Rac (1 pg/embryo) or RhoA (1 pg/embryo) mRNA. Embryos were grown to 2 dpf. Morpholino knockdown and RNA co-injection rescue experiments were done in the same clutch of embryos. The figure depicts representative embryos for every group from two independent experiments. (B) The phenotypes of all individual fish depicted in (A) were assessed by measuring tail lengths using ImageJ software. Tail lengths were depicted as a percentage of the length of non-injected control embryos. The figure represents the results of two independent experiments, where morpholino knockdown and RNA co-injection rescue experiments were performed in the same clutches of embryos. RNA co-injections were compared to their respective morpholino injections and Student t-tests were applied. * represents a P-value < 0.05, ** represents a P-value < 0.001, no asterisk represents no significant difference (P > 0.05). (C) Heterozygous ptpra+/- fish were in-crossed and offspring embryos were micro-injected at the 1-cell stage with either active Rac or active RhoA mRNA. Non-injected embryos were taken as a control. Embryos were grown to 1 somite stage and fixed. Whole mount in situ staining was performed using probes for dlx3 and hgg1. The resulting staining patterns were analyzed as described in Fig. 2H, by measuring the posterior shift of the hatching gland and the width of the dlx3 staining. After pictures were taken and phenotypes were assigned, embryos were lysed and genotyped. The average posterior shift or width of the dlx3 is plotted as a percentage of the embryo (yolk) diameter. (D) Heterozygous ptpra+/- or wildtype (WT) fish were in-crossed and their offspring was grown to 52 hpf. Embryos were characterized as mutant or sibling based on observed phenotype, dechorionated, deyolked and lysed using sodium orthovanadate and protease inhibitors. Western blots were run and probed using 5478-anti RPTPα serum, Src-pY418 antibody, Src-npY527 antibody and actin antibody as a loading control. (E) Embryos were injected with ptpra-MO (0.3 ng/embryo) or ptpre-MO (2.5 ng ptprea-MO + 2.5 ng ptpreb-MO/embryo) like in figure (A) and co-injections were done with active Fyn and Yes (4 pg each/embryo) RNA. Figure represents two separate experiments; morpholino injection and RNA co-injections were done in the same clutches of embryos. A representative embryo for each injected group is shown. (F) The lengths of the embryos′ tails were measured and the average was plotted. (G) Heterozygous ptpra+/- fish were in-crossed and embryos were micro-injected in a similar fashion as in (C) with 4 pg/embryo of both active Fyn and Yes mRNA. The embryos were stained for dlx3 and hgg1, analyzed and genotyped and the posterior shift of the hatching gland and the width of the dlx3 staining were plotted for each group. Error bars in all graphs represent standard deviations. Student t-tests were performed between indicated groups, where ** indicates a P-value < 0.001, * a P-value < 0.05 and no asterisk no significant difference (P-value > 0.05). |
| Gene: | |
|---|---|
| Antibody: | |
| Fish: | |
| Anatomical Term: | |
| Stage: | Long-pec |
Reprinted from Developmental Biology, 340(2), van Eekelen, M., Runtuwene, V., Overvoorde, J., and den Hertog, J., RPTPalpha and PTPepsilon Signaling via Fyn/Yes and RhoA is Essential for Zebrafish Convergence and Extension Cell Movements during Gastrulation, 626-639, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.