Fig. S2
- ID
- ZDB-FIG-100302-58
- Publication
- Seo et al., 2010 - BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly
- Other Figures
- All Figure Page
- Back to All Figure Page
|
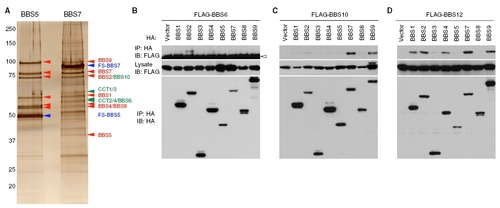
Interactions between chaperonin-like BBS proteins and BBSome subunits. (A) The BBS/CCT complex selectively associates with BBS7. BBS5 and BBS7 were tandem affinity purified from stably transfected HEK293T cells. Although purification of BBS5 copurified only BBSome subunits (red arrowheads; BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, and BBS9), purification of BBS7 resulted in copurification of the BBS/CCT complex proteins (green arrowheads; CCT1-4, BBS6, and BBS10) as well as BBSome subunits. Fusion proteins that were directly tandem affinity purified are marked with blue arrowheads. Protein identity was determined by Western blotting. It should be noted that endogenous BBS5 is absent in the FS-BBS5 sample but that endogenous BBS7 is present in FS-BBS7 sample, implying that there is only one molecule of BBS5 in the BBSome but that BBS7 may exist with different stoichiometry. (B) Interaction of BBS6 with BBS2. FLAG-BBS6 was cotransfected with BBSome subunits (BBS1, 2, 4, 5, 7, 8, 9) or BBS3, and lysates were subjected to IP. Input and immunoprecipitated amounts of each protein are shown. Open arrowhead marks IgG heavy chain. (C) Interactions of BBS10 with BBS7 and BBS9. (D) Interactions of BBS12 with BBS1, BBS2, BBS4, BBS7, and BBS9. Others are the same as in B. |