Fig. 5
- ID
- ZDB-FIG-100302-55
- Publication
- Seo et al., 2010 - BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly
- Other Figures
- All Figure Page
- Back to All Figure Page
|
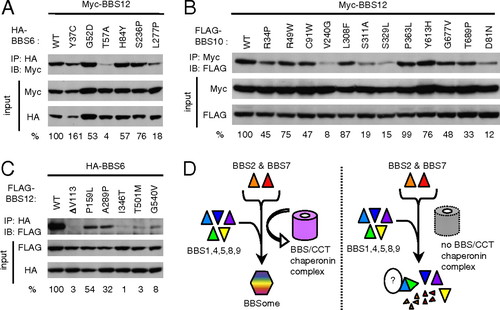
Many disease-causing missense mutations found in BBS6, BBS10, and BBS12 disrupt interactions among these proteins. (A) Interactions of BBS6 missense mutants with BBS12. HEK293T cells were transfected with Myc-BBS12 together with HA-BBS6 variants and lysates were subject to coimmunoprecipitation (IP) and immunoblotting (IB). Numbers at bottom represent ratio of coprecipitated proteins compared with wild-type protein after normalization with input. (B) Interactions of BBS10 missense mutants with BBS12. (C) Interactions of BBS12 missense mutants with BBS6. (D) Model for BBS/CCT complex function. BBS/CCT complex initially binds to BBS7 and potentially to BBS2, and mediates their association with other BBSome subunits (BBS1,4,5,8,9) to assemble BBSome. When chaperonin-like BBS genes are inactivated, at least two BBSome subunits (BBS2 and BBS7) are degraded, and the remaining BBSome subunits exist in monomeric form or aggregates with unidentified proteins. |