Fig. S1
- ID
- ZDB-FIG-081104-32
- Publication
- Laue et al., 2008 - Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development
- Other Figures
- All Figure Page
- Back to All Figure Page
|
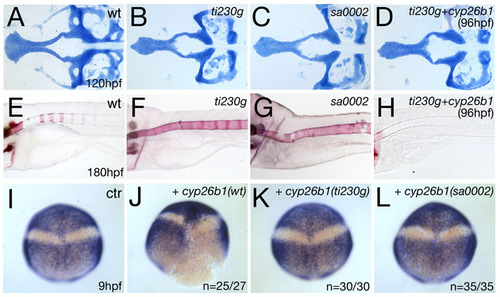
Similar effects of sa0002 and ti230 mutations and rescue of ti230 mutant by inducible application of wild-type cyp26b1. (A-H) Alcian Blue staining of craniofacial cartilaginous elements at 120 hpf (A-D) and Alizarin Red staining of bone matrix in axial skeleton at 180 hpf (E-H). (A,E) Wild-type siblings; (B,F) ti230g mutants; (C,H) sa0002 mutants. The two alleles show craniofacial and axial skeletal defects of undistinguishable strengths. (D,H) ti230 mutants after reintroduction of wild-type Cyp26b1 at 96 hpf, leading to a loss of vertebral ossification (H), whereas patterning of midline craniofacial cartilage, which occurs earlier (24-50 hpf) (compare with Fig. 7A-D), remains unaltered (D). For cyp26b1 overexpression, the heat-inducible construct pTol2-hse-GTP/cyp26b1 was generated by cloning the cyp26b1 cDNA into the bicistronic vector pSGH2 (Bajoghli et al., 2004), which in addition to cyp26b1 drives expression of GFP under the control of heat-shock elements (hse). Subsequently, the cassette was ligated into vector pT2AL200R150G, which contains Tol2 recognition sites to allow early genomic integration and widespread expression (Urasaki et al., 2006). pTol2-hse-GTP/cyp26b1 plasmid DNA was co-injected with transposase mRNA (Kawakami et al., 2004) into the cytoplasm of one-cell stage embryos from a ti230/+ intercross. For transgene activation, injected embryos were transferred from 28°C to 39°C for 30 minutes at 96 hpf. (I-L) Cyp26b1 carrying the sa0002 or ti230 mutation is biologically inactive. For activity tests, the cDNA of human CYB26B1 was amplified by RT-PCR and cloned into plasmid pCS2+. Nonsense mutations at nucleotides 136 (AAGrTAG) or 697 (TACrTAA) were introduced by PCR-based site-specific in vitro mutagenesis, yielding truncated proteins corresponding to those encoded by the zebrafish sa0002 and ti230g alleles, respectively. Plasmids were linearized with NotI, mRNAs synthesized with the Message Machine Kit (Ambion, TX) and co-injected with fluorescein dextrane (Molecular Probes) into one blastomere of wild-type embryos at the two-cell stage, as described previously (Kudoh et al., 2002). At the 90% epiboly stage (9 hpf), embryos with unilateral fluorescence were selected and fixed for in situ hybridization analysis. Whole-mount in situ hybridization for the anterior neural marker otx2 (Li et al., 1994) and the posterior neural marker hoxb1b (Alexandre et al., 1996); 90% epiboly stage, dorsal views, anterior up. To identify the injected side, some embryos were counterstained with fluorescein-coupled anti-fluorescein antibody (Molecular Probes, 1:200; not shown). (I) Uninjected sibling (n=90/90). (J) Embryo injected with wild-type CYP26B1 mRNA on right side (n=25/27). (K) Embryo injected with ti230 CYP26B1 mRNA (n=30/30). (L) Embryo injected with sa0002 CYP26B1 mRNA (n=35/35). Wild-type CYP26B1 causes loss of hoxb1b expression on the injected side, indicative of an anteriorizing effect caused by Cyp26b1-mediated RA inhibition (Kudoh et al., 2002). By contrast, neither of the mutant versions displayed any effect, suggesting that the encoded truncated proteins are inactive. |