Fig. S3
|
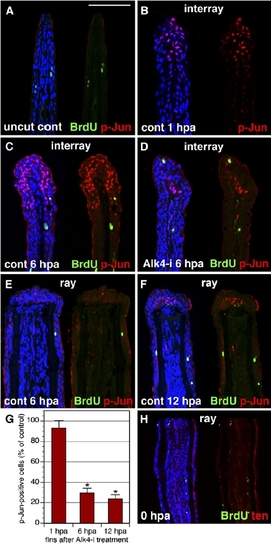
The Interray Gap Closure Is Associated with c-Jun Phosphorylation. (A–F) Longitudinal fin sections triply stained with p-Jun antibody in red, anti-BrdU in green, and ToPro3 in blue to visualize all nuclei. A presence of only few BrdU-positive cells indicates that cell proliferation does not contribute to the interray gap closure. In (A), the uncut control fin does not contain p-Jun nuclear staining. The scale bar represents 100 μm. In (B), a control fin at 1 hpa is shown. p-Jun positive cells are detected only in the wound epidermis of the interrays. No staining is observed in the proximal fin. In (C), a control fin at 6 hpa is shown (C). p-Jun antibody labels an elongated cap of epidermal cells in the distal interrays. In (D), a fin at 6 hpa treated with Alk-i inhibitor is shown. Only a few p-Jun-positive cells are identified in the distal interrays, as compared to the control (C). In (E), in contrast to the interray regions, the epidermis covering the rays does not contain p-Jun-positive cells at 6 hpa. In (F), the wound epidermis of the rays starts to acquire p-Jun-positive cells at 12 hpa (arrows). (G) Quantification of phospho-c-Jun (p-Jun)-positive cells in fins exposed to 10 mMAlk-i relative to the control, which was normalized to 100%, at 1 hpa, 6 hpa, and 12 hpa (n = 10, where two or three representative sections were analyzed from four fins). Scale bars represent the SEM. p < 0.01 indicates a significant difference between the control and inhibitor-treated fins. (H) Control fin immediately after amputation (0 hpa) stained for BrdU in green and tenascin in red. Fins were incubated with BrdU for 6 hr before fin collection. Only few scattered nuclei are BrdU positive. Tenascin staining highlights the epidermal-mesenchymal boundary in the fin. In contrast to the regenerating fins at 24 hpa (Figures 3A and 3B), mesenchyme is not labeled by tenascin-antibody staining. |