The Zebrafish Science Monitor Vol 3(5)
KEEPING AND RAISING ZEBRA FISH (DANIO RERIO) IN TÜBINGENM. Brand1, D. Beuchle1, F. Endres1, P. Haffter1, M. Hammerschmidt1, M. Mullins1, S. Schulte-Merker1, C. Nüsslein-Volhard1, R. Lück2, K. Jürgen2, and S. Schwarz2.
1Max-Planck-Institut für Entwicklungsbiologie, Spemannstr 35, 72076 Tübingen, GERMANY; 2Schwarz-Aquarienbau, Fabrikweg 8, 37075 Göttingen, GERMANY.
In the Max-Planck-Institut für Entwicklungsbiologie in Tübingen, we are running a large fish facility with about 7000 individual fish containers. Before planning the fish house, we tested many protocols, aquarium types, and procedures over a period of several years. The house HAS now run for three years, during which a very large number of embryonic mutants were identified and are being kept. Although we use a large facility (located in a separate building) that operates on the principles we describe here, we would like to emphasize that small versions of this system, where everything is located in a single room, have been built as well and run successfully in several other laboratories. The principles of the system have been described briefly (Mullins et al., 1994).
Figure 1
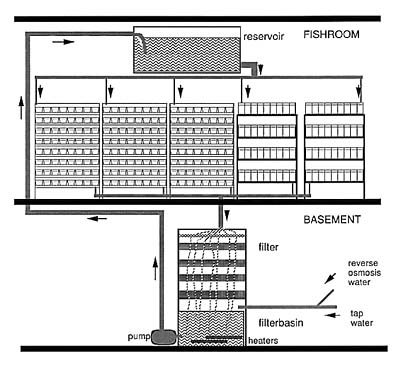
AQUARIA: In our lab, mainly concerned with mutant screens, we need a large number of rather small fish containers. These aquaria are interconnected and the water from one room with many (200-1500) aquaria is collected and recycled through one common filter per room. From the filter basin it is pumped up into a large reservoir located above the racks of aquaria in the fish room (Figure 1). From there it is distributed by gravity to the aquaria. The flow rate can be adjusted for each row of aquaria, which gives normally an exchange of 3 tank volumes per hour. In our fish house, the filters are in the basement, while in a second, small facility they lie underneath one of the racks of tanks, in the same room with the aquaria. The two different types of aquaria we use are described below (Figure 2).
Figure 2
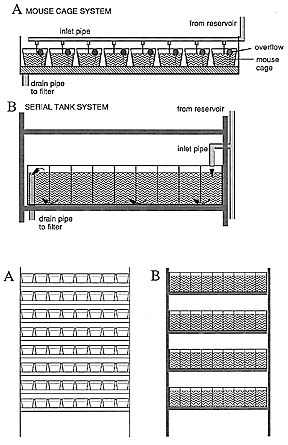
A. Overflow system (Figure 2a): Half-sized mouse cages (2.5 l) or other plastic containers are used with a hole with a grid (or a slit) that functions as an overflow. The water is supplied to these containers (7-12 on a shelf) via an overlying pipe with a series of outlets (silicone tubes with cut off eppendorf pipette tips, to ensure even water flow) through which the water squirts. The outlets are spaced such that each container can be supplied with running water (Figure 2a). The water comes from a reservoir at the top of the room, runs into each container, and exits through the overflow and onto the rimmed shelf. Three different mesh sizes (0.3 mm, 1 mm, 2 mm), depending on the age of the fish, are used to keep fish from escaping through the overflow. At one end of the shelf is a pipe extending below the shelf, where the water is collected, drained into the filter, and recycled as described above. The system holds all sizes of containers, and is therefore very versatile. One can also take out the containers and replace them in different order, or carry them away for setting up matings. We use half- or full-sized mouse cages, rat cages and small 1 liter containers for single fish and pairs of fish in this system.
Recently we have begun to use a hybrid 'serial overflow' (SO) system for keeping small numbers of fish at higher density. In the SO system, mouse cages or rat cages are further subdivided by partitionings as in the serial tank system, with slits on the bottom to allow the dirt to be carried away. SO systems stay cleaner, and seem to work well for smaller numbers of fish. This type of vessels also allows more efficient use of space; for instance, when males and females of the same genotype need to be separated, they can still reside in the same mouse cage (and they even get to see one another).
B. Serial tank system (Figure 2b): Aquaria of the dimension of about 1.20 m length, 60 cm wide and 22 cm high are subdivided by glass partitionings that leave a 1.5 mm slit at the bottom, to give a series of 8-10 interconnected compartments of about 12 liters each. Water flows into the aquarium at one end, runs through the slits, and carries the dirt with it. The water is collected at the other end by an overflow (with a cover grid to keep fish from escaping) which determines the water level. From there, water is drained into a big filter unit; clean water is then pumped up into a reservoir from which it gets distributed again, just by gravity.
Fish densities: Maximum densities are about 10 adult fish per liter of water in the serial tank system. We can raise fish to adulthood (3 month) at densities of up to 40 fish per liter in the overflow system. Usually, we transfer them into tanks after about 2 months when they are big enough not to pass through the slits anymore. The fish seem to do better at higher densities, probably because the tanks are cleaner because the water is kept in constant movement and the dirt is carried out via the overflows and the slits. Fish tend to be aggressive at low densities, so we add some plastic grass to give them a chance to hide.
Illumination: We use a 14h light-10h dark cycle. For convenience, we have lamps above every row of serial tanks; however, a sufficiently bright illumination of the room would probably suffice. The overflow systems do not have their own illumination.
Biological filters: We recycle the water through biological filters of appropriate sizes (1000 liters of water per m2 of foamed plastic of 10-30 ppi). The water runs by gravity through the filter and then into basins from which it is pumped up into reservoirs that are located above the aquaria in the fish rooms. There are two pumps per filter that run on alternate 12 h cycles; in case one breaks down, the other takes over automatically. The filters provide a large surface area for aerobic bacteria which degrade ammonium compounds to nitrite and finally nitrate. While the former compounds are very toxic to the fish, the nitrate is not. Since the surface area of the filters must be large, we use mats of foamed plastic, through which the water is dribbling into the basin, arranged in a series of shelves with increasing density of foam. The top layer is disposable synthetic filter material which collects the coarse debris; it is checked twice a week for clogging and replaced if necessary. The filter mats are cleaned twice a year in a washing machine. The production of nitrate leads to lower pH. The pH in the fish water is between 6.5 and 7.5, although higher and lower pH may work also. We measure the pH, nitrate and nitrite (using Merck sticks) in the filter basins twice a week. Normally 5% of the water is exchanged with fresh water daily; this is done using a timer. If the pH drops and/or the nitrate increase is rapid (caused by intense feeding), more fresh water is added. In addition, there is a device in the filter basin (a float like in toilets) that causes automatic refilling with fresh water in case the water level drops, because of a leak or after removal of water from the system in fish rooms (eg, for setting up crosses).
Water: Tübingen tap water is hard (approx. 500uS), very rich in CaCO3, and has a pH of 8 or higher. The composition of the tap water is as follows:
Cations mg/l Anions mg/l Calcium 73.3 Hydrogencarbonate 195 Magnesium 13.2 Chloride 17 Sodium 11.5 Sulfate 72 Potassium 2.2 Nitrate 9.7 Iron <0.01 Nitrite <0.01 Manganese <0.01 Phosphate 0.09 Ammonium <0.01
We use a mixture of tap water and reverse osmosis water, at a conductivity of about 350 uS at pH 7.5. This seems to be a good compromise between sufficiently low conductivity to stimulate egg laying and a high enough carbonate buffering capacity at an acceptable pH. All water is filtered through charcoal before going into the systems.
Temperature: The temperature of the water is adjusted to 26C with a number of heaters placed into the filter basin. The temperature in the fish rooms is slightly higher (27C) to prevent condensation of water on the walls of the rooms. Higher temperatures are uncomfortable for the researchers, they might also reduce the life span of the fish. As we have several heaters for each basin, none of which is sufficient to heat up above tolerable values, there is no danger of overheating. Temperatures dropping to room temperature by failure of the heaters is not dangerous for the fish.
Snails: We also have snails (Florida fresh water snails, Planorbella spec.) in our tanks. They clean the walls of algae and eat the surplus food, which has a very positive effect on the water quality. Adult snails are sometimes killed, and baby snails can be eaten by the adult fish, so it is necessary to regularly resupply snails. Snails multiply in the aquaria in which fish are raised. Usually some have to be removed at regular intervals. Even though snails introduce a small amount of extra work, they are very helpful; a tank without snails is easily spotted because the fish are hidden behind growing algae.
Problems with the aquaria: Clogging of the outlets, grids and slits. Snails or little pieces of dirt sometimes get into the outlets and may clog them. We check daily to ensure that the water is clean and running in all aquaria because fish kept at high density may die overnight if the water is not running. The containers in the overflow system stay clean only if there are enough fish to sufficiently stir the water such that the dirt is carried out of the overflow. With only a few adult fish and careless feeding, they may get very dirty and may need to be cleaned regularly (about once every 4 weeks). Running the water at higher rates helps, but there is a limit because the reservoirs may run dry, and the incident of clogging is higher with higher flow rates. In the serial tanks, dirt collects in compartments with no or few fish. The last compartment, from which the dirt has to exit via the overflow should therefore hold many fish (or be cleaned regularly).
Problems with the water: Tübingen tap water is too hard for good egg laying, so it has to be diluted with desalted water. On the other hand, due to its high carbonate content, it has good buffering capacity and usually the pH is stable. If the filters do not work or if their capacity is too low, the pH may rise to values above 8, preventing growth of the nitrifying bacteria in the filter. With too much feeding, this may lead to bacterial growth (turbidity) of the water. We had this problem at the beginning. Frequent water changes and adjusting the pH by mixing the tap water with reverse osmosis water at a ratio of about 1:1 will reduce this problem. In the beginning, we did not use mixed water and our charcoal filter had too low a capacity, which caused frequent and erratic death of baby fish. Strangely enough, the adult fish survived these difficult times remarkably well and even laid eggs.
Diseases: As many tanks share a common filter, there is the problem that diseases introduced into one tank may spread very quickly through the entire system. The intensive fish work carried out in our fish house (about 2,000 crosses set up weekly with fish from different aquaria and rooms, up to 200 containers of baby fish started weekly for raising) makes it very difficult to keep individual systems or rooms isolated. It would be orders of magnitude more work, and much more costly. Unlike in the case of mice and humans, fish germs do not spread via the air, so the possibilities of infection are restricted to food, water, or fish introduced from the outside. We do not use food from natural fresh water ponds. The tap water is chlorinated and goes through charcoal. We do not introduce fish from the outside without putting them into quarantine first and treating them with medicines against parasitic infections. We collect eggs from them in the quarantine room, treat the eggs with Clorox (see The Zebrafish Book for a protocol), and transfer the babies into the fish facilities.
Although we did not start our facility with a disease free population of fish, we did not have any problems with diseases for about four years. We had noticed an occasional skinny fish before, but since less than about 1/1000 of the fish were affected at any time, there was no reason for concern. In the past summer, we started observing significant numbers of sick fish, they got skinny, stopped eating and died eventually. We found that almost all were infected with nematodes (capillaria) and a few in addition with fish tuberculosis (Tb). We do not know where these diseases came from, and whether they were the primary cause of the sickness, but we assume that they were present in our population all along. The problem was more severe in the rooms where many containers held only a few old fish which had not been kept very clean. This probably led to a spreading of the infection, because the nematodes propagate via eggs passed through fish feces, that are taken up by other fish if left around. We did a round of serious cleaning of the tanks, filters and mouse cages, treated the fish with Levamisol, twice within two weeks (which was supposed to be effective against the worms, but not the Tb). The fish were mostly o.k. after this, although we did not get rid of all the worms. Now we try to remove all infected fish as soon as we detect them, and further, we try to keep the fish containers rather clean to avoid spreading the diseases via the feces of infected fish. An effective treatment to get rid of the worms was published here recently by the Driever lab, which we intend to use to solve this problem.
We have also had a serious spread of fish tuberculosis, Tb. Because there seems to be no effective treatment against Tb, we are currently starting individual rooms from sterilized eggs (see The Zebrafish Book for a protocol) after disinfecting, hoping that this will solve the problem. It will of course be ideal if the facility can be maintained disease-free in this way. According to our fish veterinarian, both diseases often occur latently in fish populations without harming them, but they can become a problem, when the fish are subjected to some form of stress. During the time we had disease problems, the fish were most certainly subjected to a significant amount of stress, as we kept setting them up frequently for crosses during our screen. Overall, therefore, our impression is that a good genetic background achieved by frequent outcrossing, healthy food, and clean conditions suppresses the level of these infections to a tolerable level.
Food: Adult fish are fed with live, frozen or dry food. We use fresh Drosophila larvae, frozen Bosmina (a small freshwater crab which can be bought here), green flakes, red flakes, and brine shrimp nauplia, for fish older than 6 weeks. Food, including dry food, is suspended in water in squirt bottles, and squirted through small holes in the lids of the tanks. Larval fish and fish for ready for mating are fed 3 times, adults twice, and adults that are not being used once daily. Baby fish are fed with salt water rotifers or paramecia during the first week of feeding, and later with brine shrimp nauplia.
Comments: We believe that a variety of different food is good for fish health. It is clear that dry food alone is inadequate to keep fish in good laying condition. Our fish like Drosophila larvae more than anything else, but growing them in large scale for fish food is no trivial task. We do not know for certain, but we suspect that the fly larvae supply the fish with essential vitamins or fatty acids they otherwise do not get in large enough quantities. Alternatively, we feed frozen Bosmina that are harvested seasonally from fish-free ponds at sugar factories; but the supply of Bosmina is limited. Artemia nauplia hatched from dry eggs are liked as well, but they are very small. Furthermore, if unhatched artemia eggs are present in the food, the fish may get seriously constipated. We cannot get live adult artemia here. Baby food always was and is the biggest problem. We tried dry baby food several times but found it quite unsatisfactory. Paramecia grown according to the protocol of the Driever lab are fine, but sometimes the cultures become contaminated (this may be a problem particular to laboratories also working with flies), together with being expensive and work intensive. For a long time we had a supply of frozen dormant rotifers, which are harvested seasonally from the ponds in sugar factories mentioned above. They hatch within 24h in fresh water and are concentrated by very low speed centrifugation. The supply, however, is limited, and presently we do not have them. A good alternative are salt water rotifers, although this requires raising the babies for some time in salt water to allow the rotifers to survive. Drosophila larvae and rotifers have the added advantage that they can be stored at 4C for two (Drosophila larvae) to 7 days (rotifers) before feeding, so they can be prepared in advance. A protocol for growing salt water rotifers is given below.
Raising fish: For egg collection, we place males and females into mating chambers (Figure 3) the preceding afternoon: A plastic box with the bottom replaced by a mesh of stainless steel (2 mm), inserted snugly into an outer box such that there is a space between the mesh net and the bottom of the outer box. We use one liter boxes for single pair matings. Plastic green grass is placed into the inner box. Five such mating chambers are held on one plastic tray, covered with a lid or with another tray (with mating boxes) (Figure 3). The water is fish water from the reservoirs. Depending on the condition of the fish, an average of 50% of single pair matings lay eggs the morning after being set up, and another 100% on the following morning. For cleanliness of the eggs, the fish are not fed on the day they are set up. They may stay in the boxes without food and water changes for two days. The shortest interval for setting up fish for mating is about one week for females, and three days for males, if the fish are to be used frequently.
Figure 3
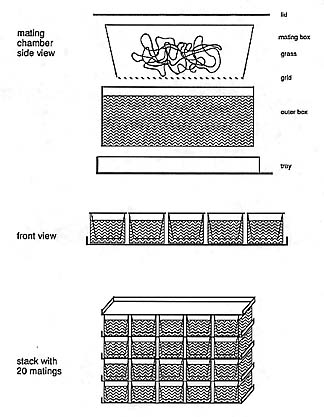
The eggs when laid fall through the steel net and are collected from the outer box with a plastic tea sieve and placed into E3 saline (5mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, 10-5% Methylene blue). We collect the good eggs and place them in petri dishes within 12 h after laying, up to 100 per 94 mm dish, or 40 per 60 mm dish in E3 medium. The water is changed once on the third day of development.
Swimming larvae (day 5) are placed into fish water (from the reservoir) at a density of 70 fish per mouse cage in about 1 liter. When salt water rotifers (which is what we usually feed now) are fed during the first week, the water is supplemented with sea salt to a concentration of 3g/l such that the rotifers survive for at least several hours. We put the babies directly into mouse cages and place the cages onto the shelves in the overflow system without connecting them to the running water. With clean paramecia or rotifers, the water does not need to be changed in the first week, otherwise it has to be replaced every other day or so. Babies are fed twice a day, such that there is always some food around. The overflow is provided with a 0.3 mm mesh sieve to prevent the babies from being flushed out. At day 14, when the baby fish are big enough to eat brine shrimp, we start feeding brine shrimp nauplia, and one snail is added per pot. We feed initially small, but increasing amounts of artemia two to three times a day, and let the water dribble through. On day 21, the fine mesh sieve is replaced with a 1 mm sieve and the flow rate is increased to slow running, and another three weeks later they are switched to the 2 mm sieve. The fish can get adult food when they are 6 weeks old or so, and can be put into tanks shortly thereafter. They may already give eggs when they are two months old, but the fecundity is optimal after 4 months or so.
Problems: For babies, the right amount of food is crucial for their survival and vigor. Heavy overfeeding with rotifers may lead to high nitrite levels, underfeeding causes unequal growth rates among the population. Overfeeding with artemia is dangerous, as rotting artemia spoil the water. A good rule of thumb is that the artemia should be mostly eaten after about 20 minutes, at which point the babies will have nice red bellies. If properly done, all 70 fish placed into a container at day five will grow up in these containers until adulthood. If space allows, baby fish can be raised at lower densities, which makes them grow even faster.
Maintenance: for 6 rooms, 5 filters with a total of about 7,000 containers holding between 1 and 100 fish ( about 300,000 fish), we have 5 full time people for cleaning the rooms and the plastic boxes, checking and cleaning the tanks, preparing the food, and feeding the adult fish. In addition, the scientific staff, the technicians and seasonal students participate in the tank checks once daily, the growing of baby food and artemia, and feeding of the individual fish and the baby fish. Control of outlets, grids, and overflows or slits from each container is done twice a day, this takes about twice two hours for one person for all 7,000 containers, plus two hours daily cleaning of slits and overflows. Every half year or so each system is cleaned thoroughly, the pipes are flushed, and the filter mats are cleaned. The coarse filters are checked daily. 5% of the water is exchanged daily, and the water measurements are made twice a week.
Safety: The systems, aquaria, shelves, filters, plumbing etc. were built by an experienced firm from high quality material (glass, valves, pipes) using a special technique for gluing the glass, rendering the aquaria extremely stable for a long time. For the aquaria, we have glass where ever possible, because it is easy to keep clean and transparent. The firm is not particularly cheap, on the other hand the great safety, stability and ease in maintenance is worth it, we believe, and, once installed, it is a very good investment. So far we have had no catastrophe such as breaking of a tank or a pipe coming loose overnight. The design allows untrained people to grasp very quickly how it works and easily take care of things. This is, we feel, of very great importance. The system is designed so the containers cannot run dry, nor can it be overheated. The worst possible catastrophe would be if both pumps of one filter unit would break down simultaneously. This would cause suffocation of the highly populated tanks, but the individual aquaria would not run dry. During the three years, we had a couple of pump breakdowns, but never both pumps in one room. There are alarm systems for pumps, temperature, water level in the reservoirs above the tanks, water level in the millipore water reservoir, and the ventilation of the rooms. These alarms are important for us because so many people use our facility. For smaller facilities, we believe that regular inspections and checks would be quite sufficient; an alarm system for the water level in the reservoirs above the tanks would probably provide adequate security.
Salt water rotifers. The following brief description is a modification of a protocol that was kindly provided to us by Drs. P. Dhert and P. Sorgeloos, University of Ghent, Belgium. A more detailed protocol is available upon request. We grow Brachionus spec. rotifers in 50 liter round plastic tanks with 15 g/l seasalt in deionized water at about 25C, with slow-bubbling aeration. Rotifers are inoculated at a density of 100-150 R/ml and are fed on instant Culture Selco food (CS, Inve Aquaculture, Baasrode, Belgium). Depending on the density of the culture, we feed increasing amounts of CS: 6 g at set up, and 6, 7, 8.5, 10, 11, 12 g on subsequent days. After 7 days, the culture has more than 500 R/ml and is harvested through a fine net, and the rotifers are resuspended in 14 liters of seawater (15 g/l sea salt), of which about a quarter is used to reinoculate a new culture. The remainder can be stored in the refrigerator for up to a week and is used for feeding the babies (2x12 ml per mouse cage per day).
Zebrafish Science Monitor Vol 3(5)
Return to Contents
