- Title
-
YTHDF2-mediated regulations bifurcate BHPF-induced programmed cell deaths
- Authors
- Lin, J., Zhan, G., Liu, J., Maimaitiyiming, Y., Deng, Z., Li, B., Su, K., Chen, J., Sun, S., Zheng, W., Yu, X., He, F., Cheng, X., Wang, L., Shen, B., Yao, Z., Yang, X., Zhang, J., He, W., Wu, H., Naranmandura, H., Chang, K.J., Min, J., Ma, J., Björklund, M., Xu, P.F., Wang, F., Hsu, C.H.
- Source
- Full text @ Natl Sci Rev
|
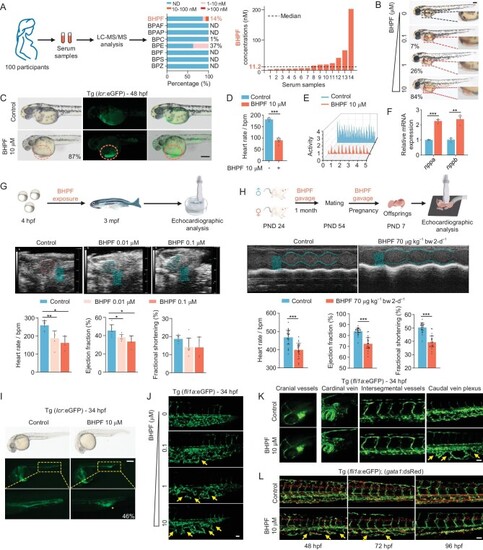
BHPF is detectable in pregnant people and displays cardiac and vascular toxicity in zebrafish and mice. A, Detection rates and concentration ranges of eight BPA substitutes in serum samples from 100 pregnant participants (left panel). Concentration of BHPF in serum samples (right panel). ND refers to not detected. B, Morphological changes of zebrafish (48 hpf) with concentration gradient exposure (0.1–10 μM) to BHPF. The pericardial condensation is indicated by red dashed boxes with percentage; |
|
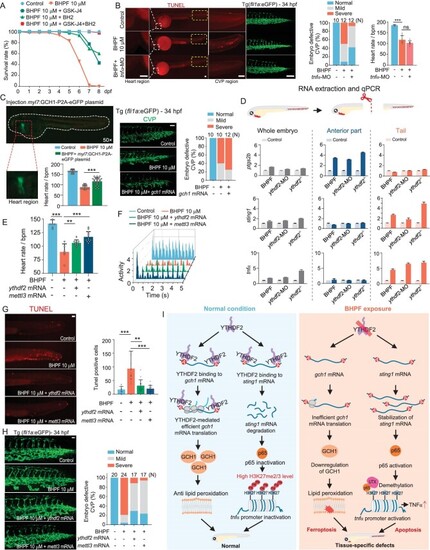
BHPF induces cardiac ferroptosis through reduction of YTHDF2-facilitated translation of m6A- |
|
BHPF-mediated downregulation of YTHDF2 induces apoptosis via activation of STING1-NFκB/UTX-TNFα axis. A and B, Effects of apoptosis inhibitor Q-VD-OPh on BHPF-induced CVP defects (A) and apoptosis (B) in 34 hpf zebrafish embryos with quantification ( |
|
YTHDF2 acts as a bifurcation point leading to tissue-specific PCDs upon BHPF exposure. A, Survival analysis of embryos upon BHPF exposure with or without cotreatment with apoptosis and ferroptosis inhibitors; |