- Title
-
Cytoglobin regulates NO-dependent cilia motility and organ laterality during development
- Authors
- Rochon, E.R., Xue, J., Mohammed, M.S., Smith, C., Hay-Schmidt, A., DeMartino, A.W., Clark, A., Xu, Q., Lo, C.W., Tsang, M., Tejero, J., Gladwin, M.T., Corti, P.
- Source
- Full text @ Nat. Commun.
|
|
|
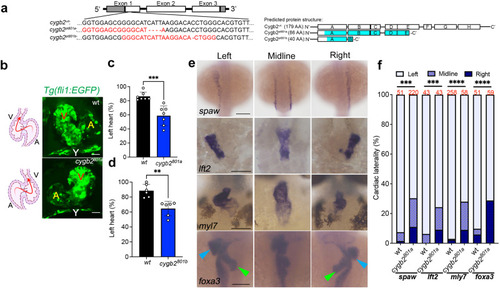
Cygb2 is expressed in cilia and through NO production regulates cilia structure. |
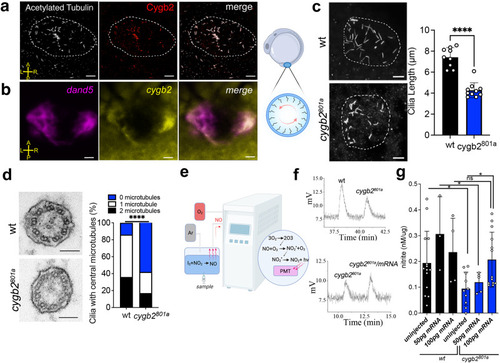
|
Perturbations of the NO signaling pathway phenocopy |
|
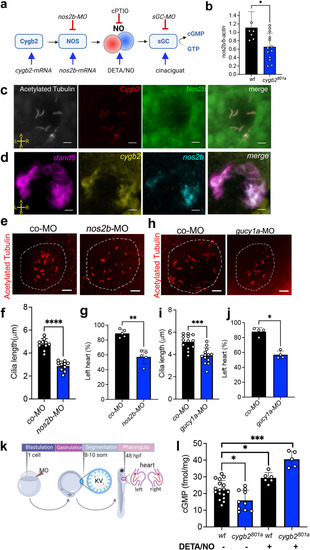
Cygb2 regulates Nos2b-NO-sGC signaling in KV cilia function and cardiac laterality determination. |
|
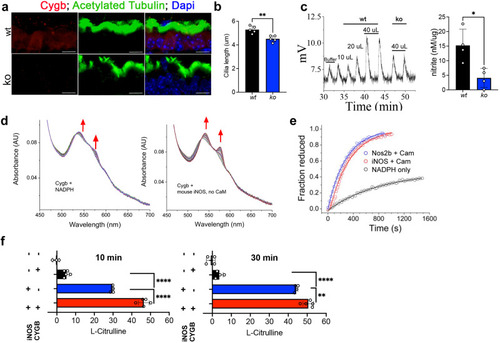
Cygb is required for NO formation through molecular interactions with iNOS. |