- Title
-
Shigella serotypes associated with carriage in humans establish persistent infection in zebrafish
- Authors
- Torraca, V., Brokatzky, D., Miles, S.L., Chong, C.E., De Silva, P.M., Baker, S., Jenkins, C., Holt, K.E., Baker, K.S., Mostowy, S.
- Source
- Full text @ J. Infect. Dis.
|
|
|
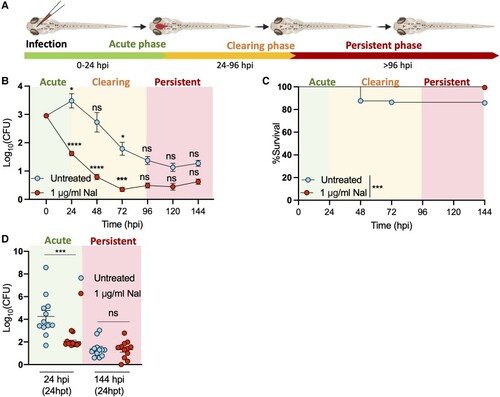
Persistent |
|
|
|
Phylogenetic distribution of persistent |
|
|
|
|