- Title
-
Bmp8a deletion leads to obesity through regulation of lipid metabolism and adipocyte differentiation
- Authors
- Zhong, S., Chen, L., Li, X., Wang, X., Ji, G., Sun, C., Liu, Z.
- Source
- Full text @ Commun Biol
|
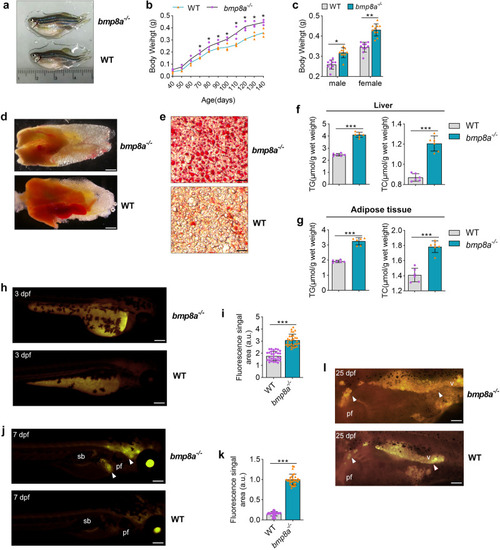
Obesogenic phenotype of |
|
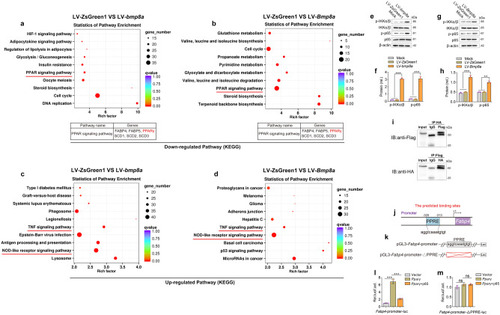
Bmp8a promotes fatty acid oxidation through AMPK and p38 MAPK pathways. |
|
Stably overexpressing zebrafish |
|
Knockdown |
|
Bmp8a activates Smad2/3 signaling to inhibit adipocyte differentiation in 3T3-L1 cells. |
|
Bmp8a activates Smad2/3 signaling to inhibit adipogenesis through type I receptor ALK4. |
|
The interaction of NF-ĸB and PPARγ mediates the effect of Bmp8a on adipogenesis. |
|
Schematic illustration of Bmp8a effect on the regulation of lipid metabolism and adipocyte differentiation. Bmp8a is required for fatty acid oxidation and regulates adipogenesis as evidenced by weight gain and fatty liver of zebrafish lacking Bmp8a. |