- Title
-
Zebrafish MAP2K7 Simultaneously Enhances Host IRF7 Stability and Degrades Spring Viremia of Carp Virus P Protein via Ubiquitination Pathway
- Authors
- Zhang, C., Lu, L.F., Li, Z.C., Han, K.J., Wang, X.L., Chen, D.D., Xiong, F., Li, X.Y., Zhou, L., Ge, F., Li, S.
- Source
- Full text @ J. Virol.
|
MAP2K7 is stimulated by virus infection. (A to H) ZFL cells seeded on 6-well plates overnight and transfected with poly I:C (1 μg/mL) (A to D) or stimulated with SVCV (MOI, 1) (E to H). At the time points 12, 24, and 48 h, total RNAs were extracted for further qPCR assays. β-actin was used as an internal control for normalization, and the relative expression is represented as fold induction relative to the expression level in control cells (set to 1). Data were expressed as mean ± SEM (n = 3). Experiments were repeated at least three times with similar results. *, P < 0.05 (compared with the control group). |
|
MAP2K7 exhibits antiviral effects both in vivo and in vitro. (A) Survival (Kaplan-Meier Curve) of wide-type (WT) and map2k7+/− zebrafish (n = 10 per group) at various days after injected i.p. with SVCV (5 × 108 TCID50/mL, 5 μL/individual). (B to D) qPCR analysis of SVCV p, n, g, and l mRNA in the spleen, liver, and kidney of WT and map2k7+/− zebrafish (n = 5 per group) given an injection i.p. of SVCV (5 × 108 TCID50/mL, 5 μL/individual) for 48 h. Each dot point represents one independent biological replicate. (E to H) Microscopy of H&E-stained heart (E), liver (F), spleen (G), and kidney (H) sections from WT and map2k7+/− zebrafish treated with SVCV (5 × 108 TCID50/mL, 5 μL/individual) for 72 h. (I and J) EPC cells were transfected with 250 ng of MAP2K7-Myc or empty vector. At 24 hours posttreatment (hpt), cells were infected with SVCV (MOI, 1) for 48 h. Then, cells were fixed with 4% PFA and stained with 1% crystal violet (I). Culture supernatants from the cells infected with SVCV were collected, and the viral titer was measured according to the method of Reed and Muench (J). (K and M) EPC cells were transfected with 2 μg MAP2K7-Myc(sh-MAP2K7) or empty vector. At 24 hpt, cells were infected with SVCV (MOI, 1). After 24 h of infection, total RNA was extracted to examine the mRNA levels of cellular p, n, g, l, and m. (L) EPC cells were transfected with 2 μg sh-MAP2K7 or empty vector. At 24 hpt, cells were infected with SVCV (MOI, 1). After 24 h of infection, total RNA was extracted to examine the mRNA levels of cellular map2k7. The relative mRNA levels were normalized to the transcription of β-actin and represented as fold induction relative to the mRNA level in control cells, which was set to 1. Data were expressed as mean ± SEM (n = 3). *, P < 0.05 (compared with the control group). |
|
Zebrafish MAP2K7 enhances poly I:C and SVCV-induced IFN activation. (A to H) EPC cells were transfected with 250 ng IFNφ1pro-Luc (ISRE-Luc) and 25 ng pRL-TK, plus 250 ng MAP2K7-HA (sh-MAP2K7) or pCMV-HA (PLKO.1). At 24 hpt, cells were untreated (null) or treated with poly I:C (1 mg/mL) or SVCV (MOI, 1). Luciferase activities were monitored at 24 h after stimulation. (I to L) Overexpression (knockdown) of MAP2K7 enhances (blocks) the expression of ifn, vig1 and isg15 induced by poly I:C (I) or SVCV (MOI, 1). EPC cells were transfected with 2 μg MAP2K7-HA (sh-MAP2K7) or empty vector for 24 h. Then the cells were transfected with poly I:C or infected with SVCV (MOI, 1) for 24 h. The total RNA was extracted to examine the mRNA levels of cellular ifn, vig1, and isg15. Data were expressed as mean ± SEM (n = 3). Experiments were repeated at least three times with similar results. *, P < 0.05 (compared with control group). |
|
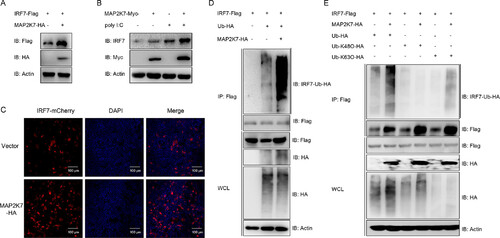
MAP2K7 is distributed throughout the cells and interacts with IRF7. (A and D) EPC cells were transfected with the indicated plasmids (5 μg each). After 24 h, cell lysates were immunoprecipitated (IP) with anti-Flag affinity gels. Then, the immunoprecipitates and cell lysates were analyzed by IB with Abs, respectively. (B) MAP2K7 is distributed throughout the cells. EPC cells were plated onto coverslips in 6-well plates and transfected MAP2K7-EGFP with and IRF7-mCherry (1 μg each). After 24 h, the cells were fixed and subjected to confocal microscopy analysis. Green signals represent the MAP2K7, red signals represent the empty vector and IRF7, and blue staining indicates the nucleus region (original magnification, ×63 oil immersion objective). Scale bars, 5 μm. (C) Schematic representation of full-length IRF7 and two mutants (IRF7-ΔC containing DBD domain and IRF7-ΔN containing the IAD domain). |
|
MAP2K7 stabilizes IRF7 by enhancing its K63-linked polyubiquitination. (A) Overexpression of MAP2K7 specifically increases the expression of IRF7. EPC cells were transfected with the indicated plasmids (1 μg each) for 24 h. (B) Overexpression of MAP2K7 increased the expression of endogenous IRF7 under the stimulation of poly I:C. EPC cells were transfected with the indicated plasmids (1 μg each). At 24 hpt, cells were transfected with poly I:C (1 mg/mL). (C) EPC cells were cotransfected with IRF7-mCherry and MAP2K7-EGFP or empty vector (1 μg each) for 24 h; then the cells were fixed and subjected to confocal microscopy analysis. Red signals represent the IRF7, and blue staining indicates the nucleus region (original magnification, ×10). Scale bar, 100 μm. (D and E) MAP2K7 increases the K63-inked ubiquitination of IRF7. EPC cells were transfected with 5 μg IRF7-Flag, 5 μg MAP2K7-HA, or empty vector and 2 μg Ub-HA, Ub-K48O-HA, or Ub-K63O-HA. At 24 hpt, cell lysates were IP with anti-Flag affinity gels. Then the immunoprecipitates and whole-cell lysates (WCLs) were analyzed by IB with the Abs. |
|
MAP2K7 associates with and degrades the SVCV P protein. (A) EPC cells transfected with the indicated plasmids (5 μg each). After 24 h, cell lysates were immunoprecipitated (IP) with anti-Flag affinity gels. (B) EPC cells seeded in 10 cm2 dishes were transfected with MAP2K7-HA (5 μg). After 24 h, the earlier cotransfected cells were infected with SVCV (MOI, 1); 24 h later, cell lysates were IP with anti-HA affinity gels. (C) EPC cells were transfected with 1 μg P-Flag and 1 μg MAP2K7-HA for 24 h. (D) EPC cells were transfected with P-Flag (1 μg) and MAP2K7-HA (0.25, 0.5, or 1 μg) for 24 h. (E) EPC cells were transfected with MAP2K7-HA. After 24 h, the cells were infected with SVCV (MOI, 1). (F) EPC cells were cotransfected with P-EGPF and vector or MAP2K7-HA for 24 h; then the cells were fixed and subjected to confocal microscopy analysis. Green signals represent the SVCV P protein, and blue staining indicates the nucleus region (original magnification, ×20). Scale bar, 50 μm. (G and H) EPC cells were transfected with 5 μg P-Flag and 5 μg P-HA (2 μg MAP2K7-Myc). After 24 h, cell lysates were immunoprecipitated (IP) with anti-Flag affinity gels. Then, the immunoprecipitates and cell lysates were analyzed by IB with Abs, respectively. Cell lysates were IP with anti-Flag affinity gels. |
|
MAP2K7 degrades the SVCV P protein by attenuating USP7-mediated K63-linked polyubiquitination. (A) EPC cells were cotransfected with the indicated plasmids (1 μg each). At 18 h after transfection, the cells were treated with dimethyl sulfoxide (DMSO), MG132 (20 mM), 3-MA (2 mM), NH4Cl (20 mM), or chloroquine (CQ) (100 mM) for 6 h before IB analysis was performed. (B) EPC cells were cotransfected with the indicated plasmids (1 μg each). At 18 h after transfection, the cells were treated with DMSO or MG132 (5, 10, or 20 mM) for 6 h. (C and D) MAP2K7 decreases the ubiquitination of the SVCV P protein. EPC cells were transfected with 5 μg P-Flag, 5 μg MAP2K7-HA or empty vector, and 2 μg Ub-related plasmids. At 18 hpt, the cells were treated with DMSO or MG132 for 6 h. Cell lysates were IP with anti-Flag affinity gels. (E and F). EPC cells transfected with the indicated plasmids (5 μg each). After 24 h, cell lysates were immunoprecipitated (IP) with anti-HA affinity gels. (G) EPC cells were cotransfected with the 1 μg P-Flag and USP7-Myc (0,0.5 or 1 μg). (H) EPC cells were cotransfected with the 1 μg P-Flag, 1 μg MAP2K7-HA, and USP7-Myc (0, 0.25, 0.5 or 1 μg). (I) EPC cells were cotransfected with the 1 μg USP7-Myc and 1 μg sh-USP7#1 (or sh-USP7#2). (J) EPC cells were cotransfected with the 1 μg P-Flag, 1 μg MAP2K7-HA, and sh-USP7#1 (0, 0.25, 0.5, or 1 μg). |
|
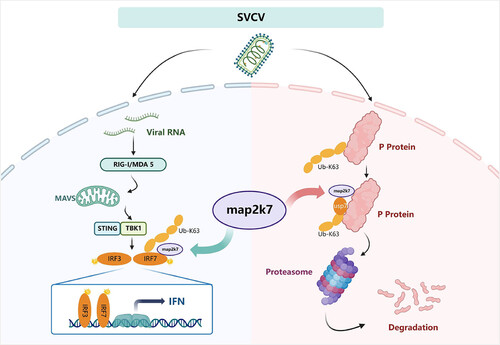
The pattern of zebrafish MAP2K7 dual regulation of antiviral immune response. On the one hand, MAP2K7 promotes the expression of IRF7, an important molecular factor of RLR pathway, by enhancing K63-linked polyubiquitination. Meanwhile, MAP2K7 degrades the P protein of SVCV by attenuating K63-linked polyubiquitination. |