- Title
-
Plasticity of dopaminergic phenotype and locomotion in larval zebrafish induced by brain excitability changes during the embryonic period
- Authors
- Bataille, S., Jalaber, H., Colin, I., Remy, D., Affaticati, P., Froc, C., Levraud, J.P., Vernier, P., Demarque, M.
- Source
- Full text @ eNeuro
|
Timing of the experiments, characterization of forebrain calcium transients and their modification by bath application of pharmacological treatments. |
|
Effects of ENE on the expression of dopaminergic markers in the zebrafish larval subpallium and olfactory bulb. |
|
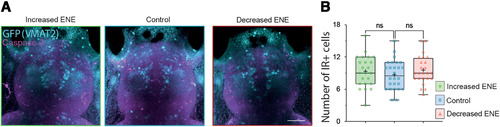
Absence of changes in programmed cell death following modifications of ENE. |
|
Effects of a 24-h bath applied treatments during embryonic development on spontaneous swimming of zebrafish larvae. |
|
Washout kinetics of the pharmacological treatments in the larval and embryonic zebrafish brain. |