- Title
-
Gap junction Delta-2b (gjd2b/Cx35.1) depletion causes hyperopia and visual-motor deficiencies in the zebrafish
- Authors
- Brown-Panton, C.A., Sabour, S., Zoidl, G.S.O., Zoidl, C., Tabatabaei, N., Zoidl, G.R.
- Source
- Full text @ Front Cell Dev Biol
|
Generation and Primary Characterization of the Gjd2b −/− /Cx35.1−/− Line. (A) General overview of the gjd2b −/− /Cx35.1−/− gene structure and partial DNA sequence. The position of forward and reverse primers targeting exon one flanked the region containing CRISPR mutations. The sgRNA target with PAM sequence is outlined by the purple box; The predicted CRISPR-Cas9 binding region targeting a XhoI restriction site is indicated by the purple arrow. The CRISPR-Cas9 genome engineering strategy produced a 1bp substitution at position 12 of exon 1, resulting in the substitution of G to A in Cx35.1−/− animals. The mutation is outlined with a green box. (B) Cx35.1 partial protein sequence. The mutation resulted in an early stop codon, as indicated by the purple asterisk (*). (C) Immunohistochemistry of gjd2b −/−/Cx35.1−/− larvae. (C1-left) A monoclonal anti-Cx36 primary antibody (Invitrogen) was applied to 6dpf wild type (TL) to determine Cx35 immunoreactivity in a frontal section of a zebrafish larva. The retina of gjd2b −/−/Cx35.1−/− fish showed fluorescence in the inner plexiform layer (IPL) and photoreceptor cell layer (PRL). The optic nerve (ON), optic chiasm (OC), and arborization fields of the retinotectal tract (AF) were immunoreactive. Scale bar 100 µm. (D, E) The immunoreactivity in the IPL and PRL of wild type TL (top) was reduced in gjd2b −/− /Cx35.1−/− larvae (bottom). Arrows indicate the IPL. Scale bars 50 µm. |
|
Shortening of the Eye Axial Length Induces a Hyperopic Shift in Adult Gjd2b/Cx35.1−/− Fish. Representative SD-OCT (A) B-mode and (B) en face images of a zebrafish eye (scale bars = 500 µm); geometrical parameters extracted from the OCT datasets are shown and annotated with different colors; the spherical lens is seen as an oval in the B-mode image (panel A) due to a significantly higher refractive index of lens compared to soft tissue. The quantification of (C) the body length (mm), (D) the lens ratio (µm/µm) as a measure of circulatiry, and (E) the retinal thickness (µm) revealed no statistically significant difference between TL controls and gjd2b −/− /Cx35.1−/−. However, the (F) axial length (from top of the lens to the RPE) normalized by body length (µm/mm), the (G) lens radius (µm), and the (H) retinal radius (from the center of the lens to the RPE; µm) collectively showed significantly smaller values (i.e., reduction in size of the eye) in gjd2b −/− /Cx35.1−/−. The (I) calculated relative refractive error (RRE; see text for definition) was significantly higher in gjd2b −/− /Cx35.1−/− which suggested a hyperopic shift, presumably, due to loss of gjd2b/Cx35.1 function. The sample size (N) was 26 for each of the TL and gjd2b −/− /Cx35.1−/− adult fish groups. |
|
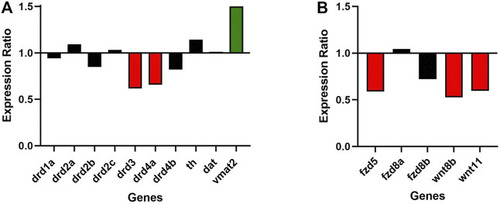
Gjd2b/Cx35.1 regulates Wnt and dopamine receptor gene expression. A qRT-PCR analysis of selected zebrafish dopamine (A) pathway genes showed the differential regulation of the D2 family receptors drd3 and drd4a, and vmat2 (N = 3). (B) Shows the downregulation of Wnt/frizzled pathway genes fdz5, wnt8b, and wnt11 (N = 4). |
|
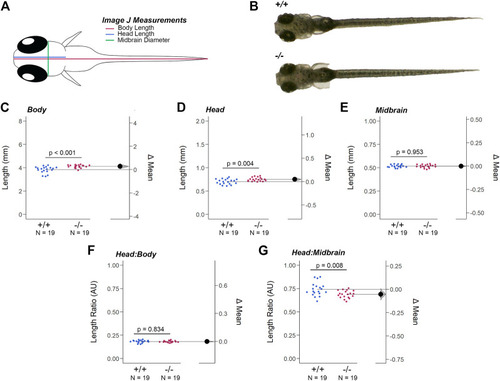
Loss of Gjd2b/Cx35.1 Caused a Dysmorphic Head Feature. (A) Illustration of the zebrafish measurements taken in Image J. (B) Images of 7dpf wild-type TL and gjd2b/Cx35.1−/− zebrafish larva. Quantification of the (C) body length, (D) head length, and (E) midbrain diameter. Gjd2b −/− /Cx35.1−/− larvae were larger in both body and head length in comparison to the age-matched wild-type larvae. The (F) Head-to-Body ratio and (G) Head-to-Midbrain ratios demonstrated a reduction in the midbrain diameter in gjd2b −/− /Cx35.1−/− larvae relative to their body size, suggesting a developmental alteration. The statistical significance was determined by the Mann-Whitney test, p ≤ 0.05. (C–G) Shows Estimation Plots. The sample size (N) was 19 for WT and gjd2b −/− /Cx35.1−/− larvae. |
|
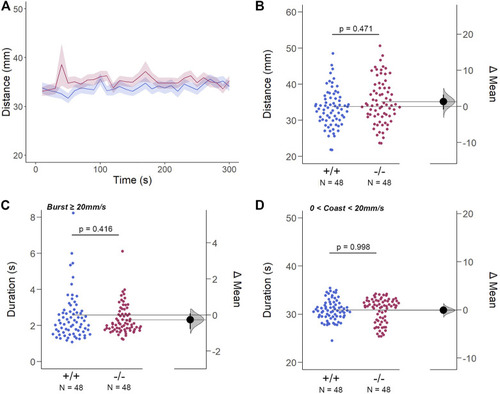
The Spontaneous Swimming Activity of Zebrafish Larvae in Continuous Light (Light-ON). (A–D) Spontaneous larval swimming activity was assessed in a free-swimming assay under light (light-ON) conditions for 5 min (A, B) The distance larvae swam (mm) lacking the gjd2b/Cx35.1 gene was indistinguishable from the WT. (C, D) The duration (s) of larval swimming bouts was assessed by analyzing the burst and coast swimming modalities separately. Gjd2b −/− /Cx35.1−/− larvae were indistinguishable from the WT. The statistical significance was determined by the Two-Way ANOVA test with the light-OFF dataset, p ≤ 0.05. The sample size (N) was 72 for WT-type and gjd2b −/− /Cx35.1−/− larvae. |
|
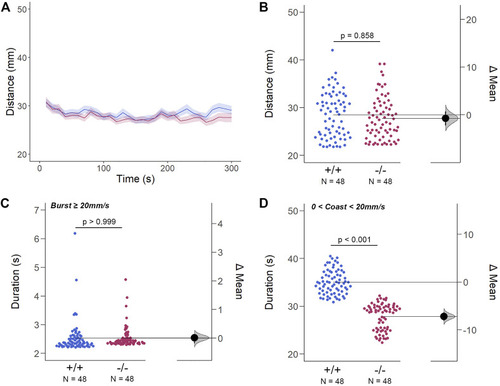
The Spontaneous Swimming Activity of Zebrafish Larvae in Continuous Dark (Light-OFF). (A–D) Spontaneous larval swimming activity was assessed in a free-swimming assay under dark (light-OFF) conditions for 5 min (A, B) The distance larvae swam (mm) lacking the gjd2b/Cx35.1 gene was indistinguishable from the WT. (C, D) The duration (s) of larval swimming bouts was assessed by analyzing the burst and coast swimming modalities separately. Gjd2 −/− /Cx35.1−/− larvae demonstrated a significant reduction in the coast duration under darkness in comparison to the WT, suggesting that dominant swimming competence was compromised in a light-dependent manner. All else was indistinguishable from the WT. The statistical significance was determined by the Two-Way ANOVA test with the light-ON dataset, p ≤ 0.05. The sample size (N) was 72 for WT-type and gjd2b −/− /Cx35.1−/− larvae. |
|
The Cone Photoreceptor Cell Activity is Enhanced in gjd2b −/− /Cx35.1−/− Larvae. (A) Overview of the experimental procedure. Burst activity was assessed for (B, C) Light-ON responses and (D, E) Light-OFF responses. Both WT and gjd2b −/− /Cx35.1 −/− larvae readily detected light transitions indicative of visual functionality. In comparison to the WT, gjd2b −/− /Cx35.1−/− larvae were hyperactive, suggesting that they were more sensitive to light stimulation. No significant difference was found for light-OFF responses. The statistical significance was determined by a Mann-Whitney test. The sample size (N) was 48 for wild-type and gjd2b −/− /Cx35.1−/− larvae. |
|
Gjd2b −/− /Cx35.1−/− Larvae Have Normal Threshold Responses to Flash Stimuli. (A) Overview of the FSTR assay protocol. Stimuli were presented in both the forward (fwd) and reverse (rv) direction to eliminate attenuation due to habituation. (B) Dark-adapted larvae were subject to light stimuli of various lengths. Average responses within 30s of the (C) 1000 m and (D) 10 m stimuli were plotted. (C, D) The mechanisms underlying visual processing speed was functionally intact in gjd2b −/− /Cx35.1−/− larvae as determined by a positive FSTR response. The statistical significance was determined by a Two-Way ANOVA test, p ≤ 0.05. The sample size (N) was 48 for WT and gjd2b −/− /Cx35.1−/− larvae. |
|
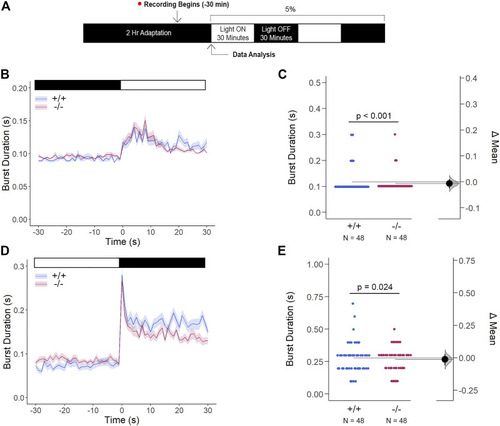
Gjd2b −/− /Cx35.1−/− Light-ON VMR Hyperactivity is Reversed with Rod Photoreceptor Recruitment via Mesopic Light Exposure. (A) General overview of the mesopic VMR Assay. Zebrafish larvae were exposed to 5% light intensity to determine their sensitivity to mesopic vision. (B–C) Light-ON responses revealed that the recruitment of rod-PRC activity reversed the burst hyperactivity previously seen in gjd2b −/− /Cx35.1−/− Light-ON VMR responses. (D–E) Light-OFF responses were reduced in gjd2b −/− /Cx35.1−/− larvae. The statistical significance was determined by the Mann-Whitney test, p ≤ 0.05. The sample size (N) was 48 for WT and gjd2b −/− /Cx35.1−/− larvae. |
|
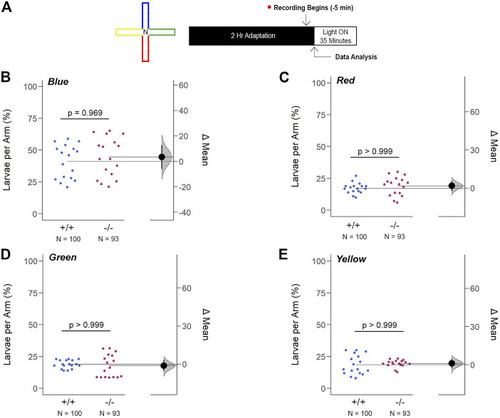
Loss of gjd2b/Cx35.1 Does Not Alter the Innate Color Preference. (A) Overview of the experimental cross-maze and protocol design used to assess innate color preference. The center of the cross maze was devoid of color and denoted as ‘N’ for neutral (B–E) Summary of the mean larvae per arm. Both genotypes equally preferred the blue arm; however, all else were indistinguishable. The statistical significance was determined by a Two-Way ANOVA test, p ≤ 0.05. The sample size (N) was 100 for WT and 93 for gjd2b −/− /Cx35.1−/− larvae. |