- Title
-
SARS-COV-2 spike protein promotes RPE cell senescence via the ROS/P53/P21 pathway
- Authors
- Zhang, Y., Peng, X., Xue, M., Liu, J., Shang, G., Jiang, M., Chen, D., Liu, B., Wang, Y., Jia, X., Xu, J., Zhang, F., Hu, Y.
- Source
- Full text @ Biogerontology
|
SARS-CoV-2 S-protein inhibits ARPE-19 cell proliferation in vitro. |
|
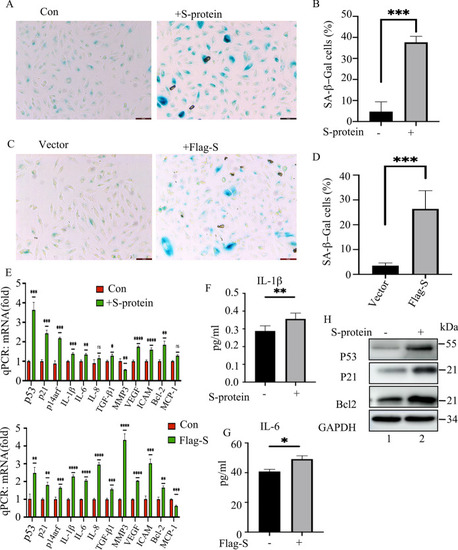
S-protein induces senescence of ARPE-19 cells. |
|
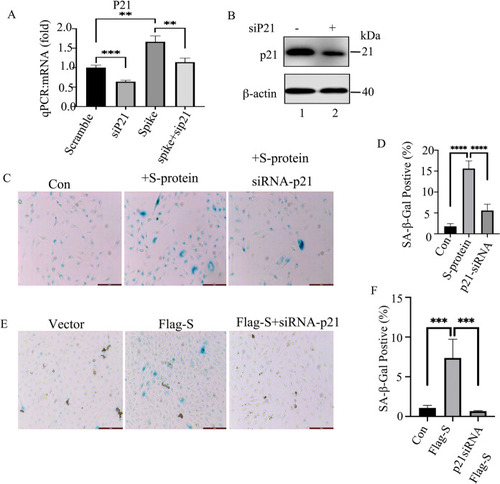
Knocking down p21 by siRNA reduces S-protein-induced cell senescence. |
|
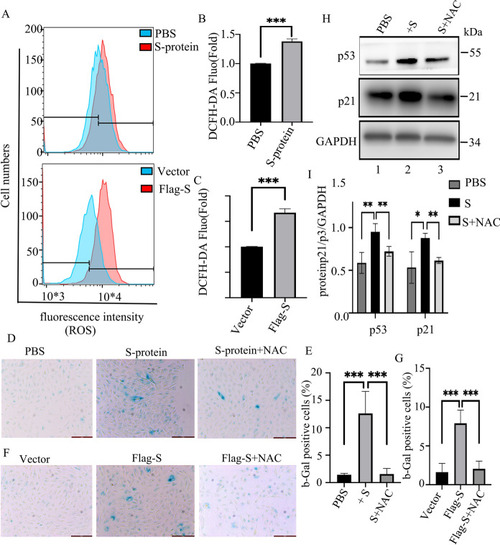
Removing ROS by NAC down-regulates S-protein-induced senescence of ARPE-19 cells. |
|
S-protein induces ER stress in ARPE-19 cells. |
|
S-protein activates NF-κB pathways in ARPE-19 cells. |
|
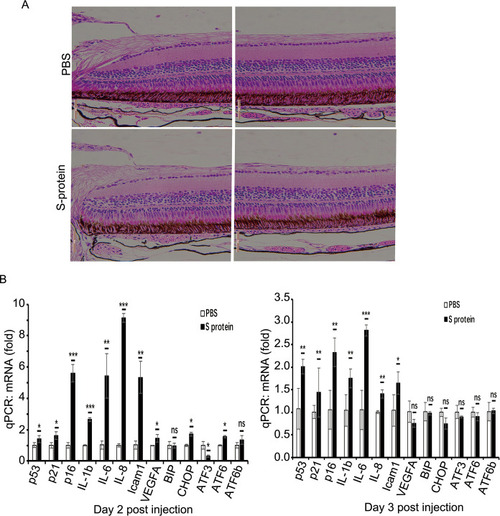
S-protein induces the expression of senescence-associated factors in the zebrafish retina. |