- Title
-
Characterization of the innate immune response to Streptococcus pneumoniae infection in zebrafish
- Authors
- Saralahti, A.K., Harjula, S.E., Rantapero, T., Uusi-Mäkelä, M.I.E., Kaasinen, M., Junno, M., Piippo, H., Nykter, M., Lohi, O., Rounioja, S., Parikka, M., Rämet, M.
- Source
- Full text @ PLoS Genet.
|
The genes were divided into functional groups based on the available gene ontology data obtained from the Ensembl genome browser versions 91 and 95, The Zebrafish Information Network (ZFIN) and literature (pie chart on the left). Immune response genes were further divided into subcategories (pie chart on the right). The numbers in the sectors indicate the number of genes in each functional group. |
|
Normalized read counts in wild type zebrafish larvae injected with KCl (unchallenged AB) or ~500 cfu of S. pneumoniae (challenged AB). The genes which had normalized read counts of ≥20 after infection and were induced by ≥3-fold are shown. Each dot represents the normalized read count of a sample of 10 larvae. The differential expressions did not reach statistical significance. |
|
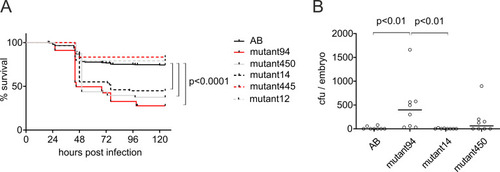
A) Survival of three mutant zebrafish lines, mutant94, mutant450, and mutant14, is compromised in pneumococcal infection compared to wild type (AB) fish and other mutant lines (mutant445 and mutant12). In the experiment, mutant and AB embryos were infected with 35–440 cfu of S. pneumoniae at 2 dpf. The graphs represent the results from three (mutant94, mutant450, mutant14, AB) or one survival experiment (mutant445 and mutant12) (n = 24–48 in each). B) Bacterial burden in mutant94 larvae is elevated after pneumococcal challenge compared to AB and other hypersusceptible mutant lines. Embryos were infected with 505 cfu of S. pneumoniae at 2 dpf and bacterial counts were determined at 18 hpi. The circles represent bacterial counts in a single larva from one experiment (n = 8). Median bacterial count is depicted with a line. The statistical comparison of difference was calculated with log-rank (Mantel-Cox) test in A, and with Kruskal-Wallis test with Dunn’s multiple comparisons test in B. cfu = colony forming units. |
|
The figure shows normalized read counts for crp2-1, il1b, il6, and tnfa in wild type larvae injected with KCl (unchallenged AB), wild type larvae infected with ~500 cfu of S. pneumoniae (challenged AB), and mutant94 larvae infected with ~500 cfu of S. pneumoniae (challenged mutant94) at 18 hpi. Each dot represents the normalized read count in a sample of 10 larvae. The differential expressions did not reach statistical significance. |
|
A) A schematic presentation of the zebrafish |
|
The figure shows the survival of wild type (WT (crp2tpu6)), heterozygous (crp2tpu6/+) and homozygous (crp2tpu6/tpu6) larvae after pneumococcal infection. In the experiment, 1,646 cfu of S. pneumoniae was injected into the bloodstream of 2 dpf larvae and the survival was monitored until 5 dpf. Data were collected from a single experiment with the group sizes (n) indicated in the figure. The statistical comparison of difference was calculated with log-rank (Mantel-Cox) test. |
|
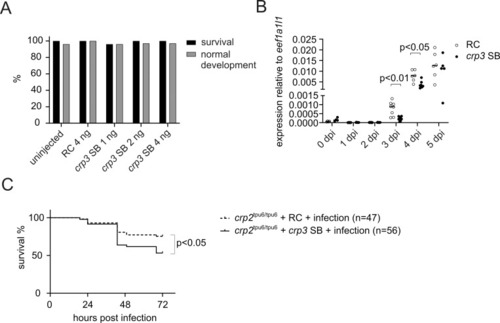
A) Percentage of surviving and normally developing uninjected zebrafish larvae, larvae injected with 4 ng of RC morpholino, and larvae injected with different doses (1 ng, 2 ng or 4 ng) of crp3 SB morpholino. In the experiment, RC or crp3 SB morpholino was microinjected into the yolk sac of AB zebrafish embryos at the 1–4 cell stage and their survival and development was followed for 5 days. The percentage represents the live (black) and morphologically normal (gray) larvae in each group (n = 31–50) at 5 dpi. B) crp3 transcript levels in RC morpholino and crp3 SB morpholino injected AB zebrafish larvae at 0–5 dpi. In the experiment, 4 ng of RC or crp3 SB morpholino was microinjected into the yolk sac of AB zebrafish embryos at 1–4 cell stage and pools of 3–5 larvae (n = 7–8) were collected at specific time points. crp3 expression levels were measured with qPCR from technical duplicates and normalized with eef1a1l1 expression. A dot/circle represents the relative gene expression in a single pool of embryos/larvae, which was calculated using the 2-ΔCt method, and the line depicts the median expression level. C) The survival of RC or crp3 SB morpholino injected homozygous (crp2tpu6/tpu6) larvae after pneumococcal infection. In the experiment, 4 ng of RC or crp3 SB morpholino was microinjected into the yolk sac of crp2tpu6/tpu6 embryos of F3 generation at 1–4 cell stage, and at 2 dpi the larvae were infected with 296 cfu of S. pneumoniae. The survival of infected larvae was monitored until 5 dpf. Data were collected from a single experiment, with the group sizes (n) indicated in the figure. The statistical comparisons of difference were calculated with Kruskal-Wallis test with Dunn’s multiple comparison in B, and with log-rank (Mantel-Cox) test in C. dpi = days post injection. |