- Title
-
OligoTRAFTACs: A generalizable method for transcription factor degradation
- Authors
- Samarasinghe, K.T.G., An, E., Genuth, M.A., Chu, L., Holley, S.A., Crews, C.M.
- Source
- Full text @ RSC Chem Biol
|
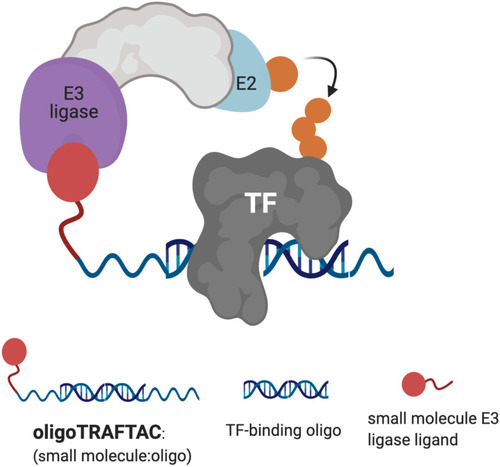
Schematic representation of oligoTRAFTAC-mediated transcription factor (TF) and E3 ligase recruitment, and proximity-dependent TF ubiquitination. |
|
OligoTRAFTAC induces c-Myc degradation. (A) The oligonucleotide selected for the c-Myc oligoTRAFTAC engages c-Myc. HeLa cell lysates were incubated with biotinylated oligonucleotide, or its scrambled sequence, followed by capture with streptavidin agarose and probing for c-Myc. (n = 2) (B) Dose response of OT7-mediated c-Myc knockdown in HeLa cells. (n = 2) (C) OligoTRAFTAC-induced c-Myc degradation occurred via the proteasomal pathway. HEK293T cells were treated with c-Myc-targeting oligoTRAFTAC with and without the neddylation inhibitor, MLN-4924 (1 μM), and then analyzed for c-Myc levels. (n = 2, *p < 0.05) (D) HEK293 cells were preincubated with and without 10 μM VHL ligand followed by OT7 transfection and analyzed for c-Myc levels. (n = 2, *p < 0.05) (E) Chemical structure of the c-Myc-targeting oligoTRAFTAC (OT7). |
|
Brachyury-GFP degradation by oligoTRAFTACs. (A) Brachyury-targeting oligonucleotide used in the oligoTRAFTAC design engaged with brachyury-GFP. Brachyury targeting biotinylated oligonucleotide (BRCH) or its scrambled oligonucleotide (SCRM) incubated with cell lysate and captured by streptavidin agarose beads. (n = 2) (B) Two oligoTRAFTACs with 3′ VHL ligand modifications, OT3 (5 PEG unit linker) and OT4 (2 PEG unit linker) were transfected into HEK293T cells and brachyury-GFP levels were analyzed in lysates prepared after 20 h. (n = 2) (C) OT3 induced brachyury-GFP degradation as early as 12 h in HEK293T cells. (n = 2) (D) Washout experiment after 12 h of OT3 transfection. Cells were incubated continuously for 24 h in transfection medium or OT3 was aspirated after 12 h of transfection and fresh medium added to cells. Washout cells incubated for another 12 h and 24 h prior to harvesting. (n = 2) (E) Chemical structure of brachyury-targeting oligoTRAFTAC (OT3). OT3 consists of a phosphodiester backbone. |
|
OligoTRAFTACs induce brachyury-GFP degradation via the proteasomal pathway. (A) HEK293T cells expressing brachyury-GFP were transfected with 75 nM each of OT3 and its scrambled OT6, and lysates were probed for brachyury levels. (n = 2, **p < 0.005) (B) OT3 induced brachyury degradation is VHL-dependent. HEK293T cells were preincubated with and without 10 μM of VHL ligand for 1.5 h prior to OT3 transfection. After 20 h of transfection, cells lysates were prepared and analyzed for brachyury degradation. (n = 3, ****p < 0.0001) (C) OT3 induces brachyury degradation via the proteasomal pathway: neddylation inhibitor MLN-4924 was preincubated with cells prior to OT3 transfection. After 20 h of transfection of OT3, cells were harvested and analyzed for brachyury levels. (n = 2, **p < 0.01) (D) Brachyury-GFP downregulation was monitored by GFP fluorescence in cells in the presence or absence of MLN-4924. (n = 2). |
|
Endogenous brachyury degradation by oligoTRAFTACs constructed with phosphorothioate backbone. (A) Increasing concentrations of OT17 were transfected into UM-Chor1 cells and harvested after 24 h, subjected to lysis and analyzed for brachyury downregulation. Brachyury levels were normalized to loading control and presented as a bar graph. (n = 2, **p < 0.01) (B) JHC-7 cells were transfected with OT17 and probe for brachyury levels (n = 2, ****p < 0.0001). (C) UM-Chor1 cells were transfected with 60 nM of OT17 and harvested at subsequent different time points as indicated. (n = 3, ****p < 0.0001) (D) Washout experiment: transfection medium was removed after 12 h of OT17 transfection and UM-Chor1 cells were incubated for another 12 h or 24 h in fresh complete cell culture medium. (n = 2, ***p < 0.001, ****p < 0.0001) (E) OT17-mediated brachyury degradation is oligonucleotide sequence dependent. UM-Chor1 cells were transfected with OT17 and scrambled OT20, cells were lysed and analyzed as shown. (n = 2, ***p < 0.001, ****p < 0.0001) (F) OT3- and OT17-induced brachyury ubiquitination. HEK293T cells that overexpress brachyury-GFP were transfected with HA-ubiquitin, followed by the second transfection with OT3 or OT17. After 12 h, cell lysates were subjected to immunoprecipitation using brachyury antibody, and the eluates blotted for the indicated proteins. (n = 3). |
|
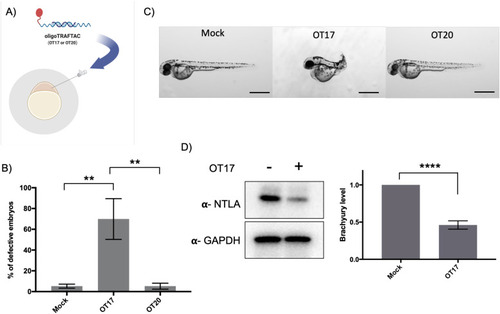
Microinjection of brachyury-targeting oligoTRAFTAC into zebrafish embryos: demonstration of in vivo activity. (A) Schematic representation of OT17 and OT20 microinjection into zebrafish embryos. (B) Quantitation of the defective embryos in mock, OT17 and OT20 injected groups. Mock, OT17 and OT20 (180 picoliters from 25 μM of oligoTRAFTACs, or mock equivalent) were microinjected into embryos (number of embryos in each group for three independent experiments; mock-47, 50, 43; OT17-49, 52, 61; OT20-75, 74, 45). After 48 h, the number of defective tails in each group was recorded and presented as percentage in a bar graph. (n = 3, **p < 0.001) (C) Images of representative zebrafish from the cognate treatment groups. Pictures were captured after 48 h post microinjection of mock, OT17 and OT20. Scale bar 500 μm. (n = 3) (D) Brachyury levels in zebrafish embryos after OT17 (180 picoliters from 25 μM of oligoTRAFTACs, or mock equivalent) injection. Embryos were collected at 8–10 somite stage, subjected to lysis, and probed for brachyury levels. (n = 3, ****P < 0.0001). |