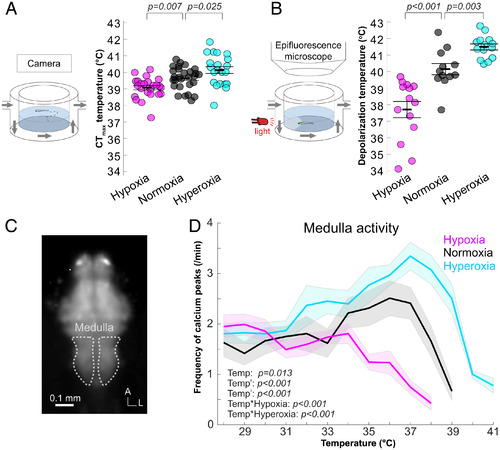
Oxygen availability affects both CTmax and onsets of neural dysfunctions. (A) Effect of oxygen availability on CTmax measured in freely swimming fish (setup illustrated in Left panel) during heat ramping with water oxygen level of 60% (hypoxia; magenta circles; n = 24), 100% (normoxia; black circles; n = 28), or 150% (hyperoxia; cyan circles; n = 21) of air-saturated water (SI Appendix, Table S7). (B) Brain-wide depolarization-onset temperatures measured in agar-embedded, Tg(elavl3:GCaMP6s) 5-d-old fish (setup illustrated in Left panel) during heat ramping in hypoxia (n = 14), normoxia (n = 12), and hyperoxia (n = 14) (SI Appendix, Table S8). It is important to bear in mind that CTmax (A) and neural (B) data were collected in freely swimming versus embedded larvae, respectively, and that direct comparisons of these temperatures should be interpreted with caution. (C) Image outlining the medulla where the frequency of calcium peaks was measured. A, anterior; L, lateral. (D) Frequency of medulla calcium peaks during heat ramping in hypoxia (n = 12), normoxia (n = 10), and hyperoxia (n = 11) (SI Appendix, Table S9). (A, B, D). Results for hypoxia (60%; magenta), normoxia (100%; black), and hyperoxia (150%; cyan) are presented with mean and SE (A and B: bars and error bars; D: solid lines and shaded area). Temp, temperature.
|