- Title
-
Efficacy of Tricaine (MS-222) and Hypothermia as Anesthetic Agents for Blocking Sensorimotor Responses in Larval Zebrafish
- Authors
- Leyden, C., Brüggemann, T., Debinski, F., Simacek, C.A., Dehmelt, F.A., Arrenberg, A.B.
- Source
- Full text @ Front Vet Sci
|
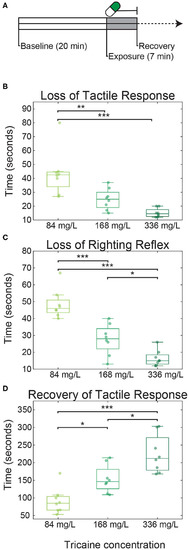
Tricaine onset and recovery in non-restrained zebrafish larvae. |
|
Effects of tricaine and gradual cooling on the heartrate. |
|
Figure 3. Effects of tricaine and gradual cooling on the optokinetic response. (A) Illustration of the visual stimulus (top) and the experimental protocol (bottom). A 3 min looping stimulus was presented to animals using an LED arena (Supplementary Figure 1). It consisted of a moving bar rotating at a constant velocity (18°/s) for 60 s counter-clockwise, followed by 60 s clockwise, and finally alternating between counter-clockwise and clockwise in 4 s intervals for a total of 60 s. (B) Sample traces of the eye movements evoked by the stimulus protocol during experiments carried out in the presence of the vehicle control (1% v/v 1,2-propanediol), during gradual cooling (11°C) and in the presence of tricaine at a concentration of 168 mg/L. (C,D) The dynamic range of the evoked eye movements and the number of saccades occurring during each 3 min stimulus period were analyzed and significance was determined via two-way repeated measures ANOVA with Tukey's HSD test. Horizontal colored bars indicate which time points were significantly different from respective controls, for clarity only one significance level is shown (p < 0.05). Vertical white lines indicate the 1 min stationary grating period shown in (A), as no visual stimulus was present during this time. (E) Comparison of the recovery timepoints of saccade rate and dynamic range for the different treatments. N = 4–18. |
|
Gradual cooling induced a calcium surge in both the pretectum and hindbrain of exposed larvae. |
|
Effects of tricaine and gradual cooling on sensory and motor brain areas. |
|
Reduced sensorimotor activity in pretectum and hindbrain after application of tricaine and gradual cooling anesthesia. |
|
Schematic illustrating the differential effects of 168 mg/L tricaine and 11°C cooling on physiological parameters and the optokinetic response, as well as their respective time courses. |