- Title
-
A Markerless Pose Estimator Applicable to Limbless Animals
- Authors
- Garg, V., André, S., Giraldo, D., Heyer, L., Göpfert, M.C., Dosch, R., Geurten, B.R.H.
- Source
- Full text @ Front. Behav. Neurosci.
|
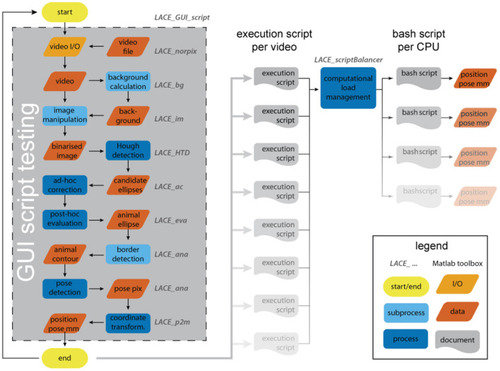
The analysis flow of LACE. The user interacts with most toolboxes through a graphical user interface (GUI). The GUI results in an execution script that holds all information and file positions to run an analysis on the entire video. By testing the script inside the GUI, the system is able to calculate the analysis duration, which is used in the computational load management. The bash scripts can be run over night. |
|
Image Manipulation. |
|
These are illustrations of five standard problems |
|
Schematic overview of the pseudo skeleton calculation. The figure illustrates the general procedure used to derive a pseudo-skeleton, therefore all vertices, contours, etc. are schematic drawings and not based on data or results of the algorithms. The pose detection uses the center of the ellipse detected by |
|
A histogram of the correction frequency per frame for 1,318 different zebrafish video. 1,176 videos needed no correction at all. In 107 videos, less than 5% of the frames were corrected. Note that the counts are depicted on a logarithmic scale. Above the histogram bars, a rug plot (similar to a scatter plot) of the occurrences is given. Each vertical marker represents a video at the given correction frequency on the x-axis. |
|
Quantification of body peristaltic contractions of freely crawling |
|
An example trajectory of an adult zebrafish traced with LACE. |
|
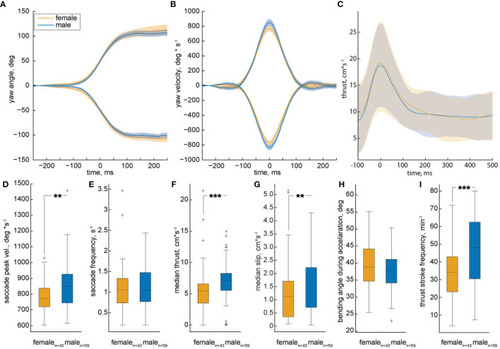
Analysis of multiple trajectories by female and male zebrafish during motivated trials. The median yaw angle |