- Title
-
Insights Into the Peroxisomal Protein Inventory of Zebrafish
- Authors
- Kamoshita, M., Kumar, R., Anteghini, M., Kunze, M., Islinger, M., Martins Dos Santos, V., Schrader, M.
- Source
- Full text @ Front. Physiol.
|
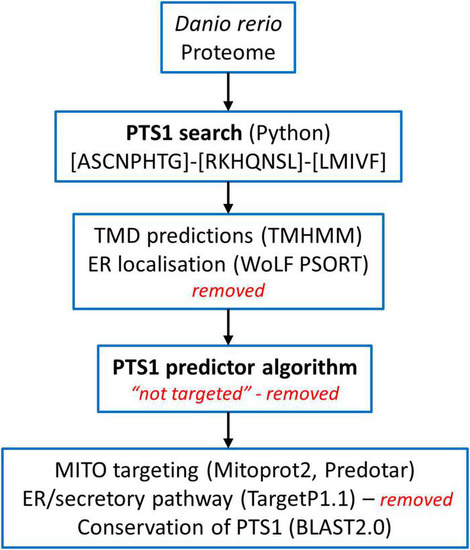
Overview of the screening of the |
|
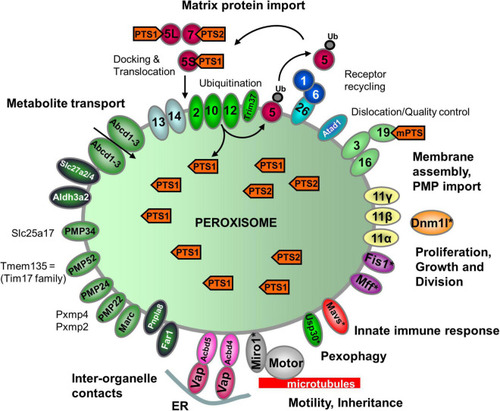
Schematic overview of the predicted molecular machineries and |
|
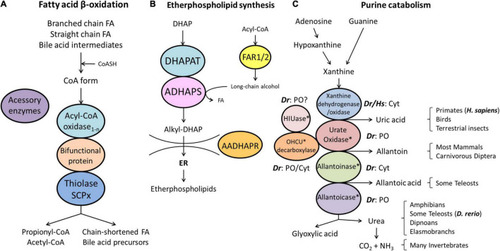
Schematic representation of the pathways of fatty acid β-oxidation |
|
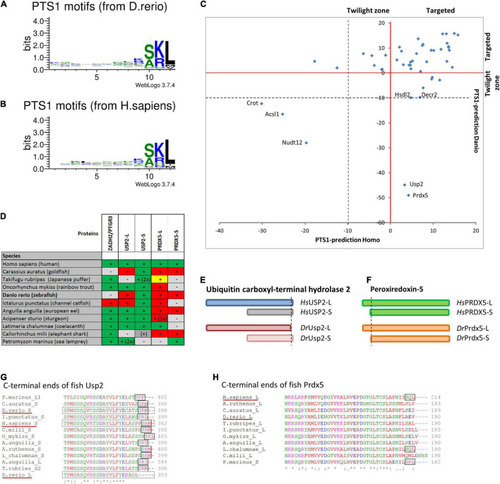
Comparison of PTS1 motifs between |
|
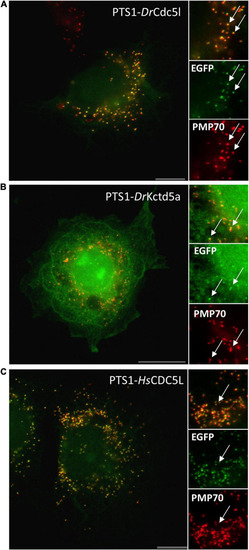
Verification of PTS1 functionality for selected candidate proteins. COS-7 cells were transfected with an EGFP fusion protein containing the C-terminal putative PTS1 of |
|
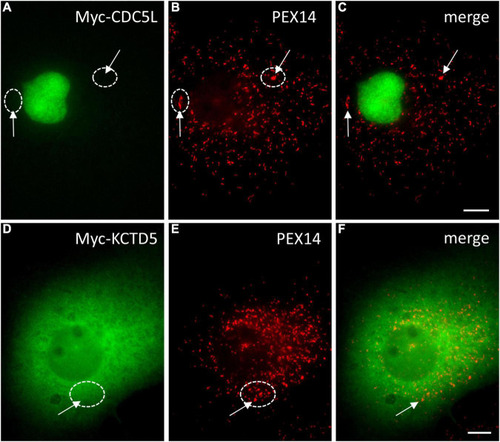
Localisation of |