- Title
-
Collagen fibers provide guidance cues for capillary regrowth during regenerative angiogenesis in zebrafish
- Authors
- Senk, A., Djonov, V.
- Source
- Full text @ Sci. Rep.
|
Vascular organization of intact caudal fin vs. early regeneration. Organization of tissue compartments in the intact caudal fin ( |
|
Differences in tissue compartments between the intact (unamputated) caudal fin and fin during early regeneration. Structural differences between intact caudal fin ( |
|
In vivo effects of the collagen cross-linking inhibitor, BAPN, on caudal fin regeneration and neoangiogenesis. Vascular alteration during normal fin regeneration ( |
|
Collagen cross-linking inhibitor BAPN causes structural changes in the collagen content and blood vessel formation. |
|
Collagen cross-linking inhibitor BAPN affects collagen I, II and IV accumulation, appearance and distribution. Double immunofluorescence staining revealed the relationship between the new formed blood vessels and collagen I, II, IV distribution in control (left panel: |
|
Ablation of collagen 1α2 producing cells by NFP impair caudal fin regeneration and vascular development. In the control animals, at 3dpa and 7dpa, classical tissue regeneration pattern and blood vessel morphology are documented ( |
|
NFP treatment causes collagen deficiency and impairment of caudal fin regeneration. Electron microscopy performed on longitudinal sections revealed severe structural alteration after NFP treatment. 3dpa ( |
|
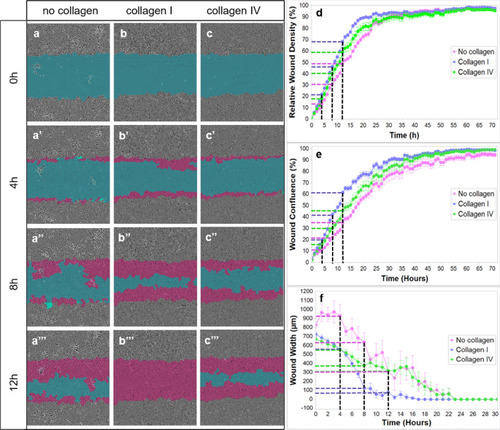
In vitro ECs migration on collagen I and collagen IV substrates. The blue region denotes the scratch wound mask over time (0 h, 4 h, 8 h, and 12 h). ECs migrate into the wound area on non-coated ( |
|
Collagen cross-linking inhibition and reduced collagen 1α2 impair blood vessel formation. Vascular development (ECs - green) in the zebrafish embryo at 5dpf in a control animals ( |