- Title
-
Influence of Mpv17 on Hair-Cell Mitochondrial Homeostasis, Synapse Integrity, and Vulnerability to Damage in the Zebrafish Lateral Line
- Authors
- Holmgren, M., Sheets, L.
- Source
- Full text @ Front. Cell. Neurosci.
|
Mitochondrial homeostasis in WT and |
|
|
|
FM1-43 uptake is reduced in |
|
|
|
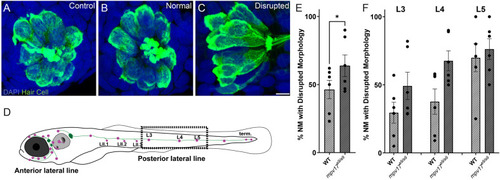
Mechanical overstimulation results in morphological disruption of neuromasts more frequently in |
|
WT and |
|
Both WT and |
|
Figure 8. PSDs are enlarged in mpv17a9/a9 neuromasts but do not significantly change upon mechanical overstimulation. (A–D) Maximum-intensity projections of confocal images of WT (A,B) and mpv17a9/a9 (C,D) neuromasts, either unexposed (A–C) or exposed to strong water current (B,D). (E) Quantification of presynaptic ribbon volume relative to unexposed WT control. n = 1013–1087 presynaptic particles; N = 7 experimental trials. (F) Quantification of PSD volume relative to WT control. PSDs are enlarged in unexposed mpv17a9/a9 neuromasts and mechanically overstimulated WT neuromasts, but there is no significant change in PSD size in mechanically overstimulated mpv17a9/a9 neuromasts (****P < 0.0001 WT exposed; *P = 0.0100 mpv17a9/a9 control). n = 486–683 PSD particles from neuromasts L3, L4, and L5; N = 5 experimental trials. Scale bar: 5 μm. Whiskers: min. to max. values; “+” indicates mean value. “ns” = not significant. |