- Title
-
VEGFC/FLT4-induced cell-cycle arrest mediates sprouting and differentiation of venous and lymphatic endothelial cells
- Authors
- Jerafi-Vider, A., Bassi, I., Moshe, N., Tevet, Y., Hen, G., Splittstoesser, D., Shin, M., Lawson, N.D., Yaniv, K.
- Source
- Full text @ Cell Rep.
|
Endothelial cells sprout from the PCV in G1 phase (A) Schematic representation of cell cycle stages in ECs as highlighted by the (B–E) Selected confocal snapshots from a time-lapse series of a (F) Fraction of late-G1 ECs out of total PCV cells (n26 hpfembryos = 11; n42hpf embryos =9 ). Data show mean ± SEM (unpaired t test). (G) Spatial distribution of late-G1 ECs in the PCV at 26 and 42 hpf (n26 hpf embryos = 11; n42 hpf embryos =9 ). Data show mean ± SEM (unpaired t test). (H–O) Selected snapshots from a time-lapse series of a (P–S) Confocal images of 30 (P and R) and 48 (Q and S) hpf (T and U) Spatial distribution of p53+ (n30 hpf = 7, n48 hpf = 4) (T) and p27+ (n30 hpf = 18, n48 hpf = 8) (U) ECs in the PCV. Data show mean ± SEM (one-way ANOVA plus Tukey`s post-hoc test). Scale bars: 40 μm (B–E and H–O), 70 μm (P–S); ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant. |
|
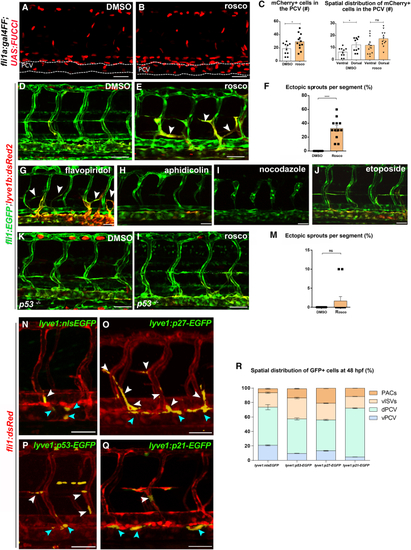
G0/G1 cell-cycle arrest in PCV ECs is mediated by p53, p21, and p27 (A–C) Confocal images of (D and E) Confocal images of 48-hpf (F) Number of ectopic sprouts per segment in roscovitine- versus DMSO-treated embryos (nDMSO = 11, nrosco = 12). Data show mean ± SEM (unpaired t test). (G–J) Confocal images of 3-dfp (K and L) Confocal images of (M) Percentage of ectopic sprouts per segment in roscovitine-treated (n = 12) versus DMSO-treated (n = 12) (N–P) Confocal images of (Q) Confocal images of (R) Spatial distribution of GFP+ cells in |
|
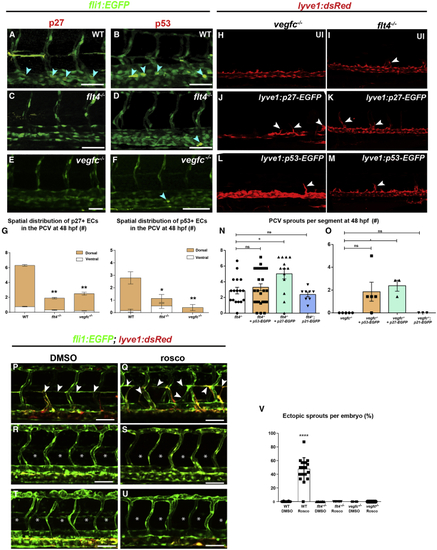
Cell-cycle arrest in dorsal PCV ECs is VegfC/VegfR3 dependent (A–F) Confocal images of 48-hpf WT (A and B), (G) Number and spatial distribution of p27- and p53-stained ECs, in the PCV of WT, (H–M) Confocal images of 48-hpf (N) Number of ectopic sprouts per segment in (O) Number of ectopic sprouts per segment in (P–V) Confocal images of WT (P and Q), |
|
Cell-cycle arrest acts downstream to ERK signaling in PCV ECs (A–E) Confocal images of 48-hpf (F–L) Confocal images of (M–P) Confocal images of 34-hpf |
|
(A–C) In situ hybridization at 24 and 30 hpf showing reduced expression of lyve1 (A) and nr2f2 (B) following roscovitine treatment. ephb4a expression remains unchanged (C). (D and E) Confocal images of 28-hpf Tg(fli1:EGFP;prox1a:kalt4:UAS:uncTagRFP) embryos showing reduced numbers of prox1+ cells (light-blue arrowheads) in the PCV upon roscovitine treatment. Co-localization channel is shown in yellow; quantified in (F). Data show mean ± SEM (unpaired t test). (G–L) Selected images from a time-lapse series depicting the dynamics of PCV sprouting in the presence of roscovitine. Two ECs (colored in red and blue) leave the PCV as part of a single sprout (G) that generates two vessel types. The leading EC (red) connects to an arterial ISV to generate a vISV (I and J); the following cell (blue) divides (J) and gives rise to 2 daughter cells (K and L, yellow arrowheads), with one joining a vISV and the second one incorporating into the nascent PAC. (M) Schematic model depicting cellular events inducing cell-cycle-arrest-induced sprouting. Dorsal cells of the PCV sense high levels of Vegfc (purple) secreted from the hypocord and the DA. Activation of Vegfr3/Flt4-ERK signaling in these cells leads to cell-cycle-arrest-induced sprouting. |