- Title
-
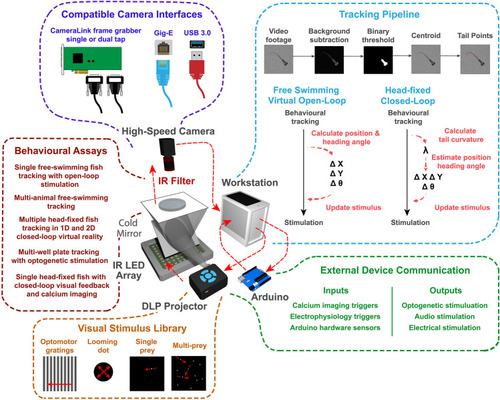
BonZeb: open-source, modular software tools for high-resolution zebrafish tracking and analysis
- Authors
- Guilbeault, N.C., Guerguiev, J., Martin, M., Tate, I., Thiele, T.R.
- Source
- Full text @ Sci. Rep.
|
Overview of the behavioral feedback system. The behavioral feedback hardware consists of modular components based on a previous design |
|
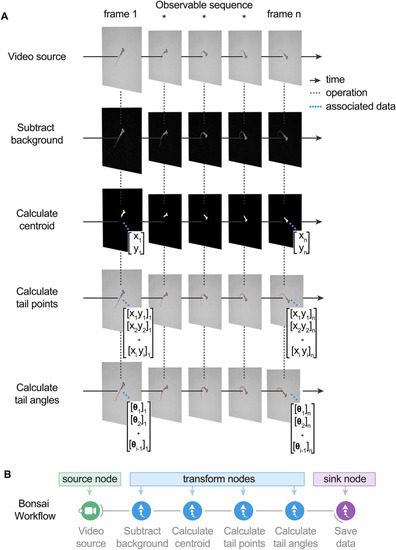
BonZeb inherits Bonsai's reactive architecture for processing data streams. ( source node generates images over time. The video source can either be a continuous stream of images from an online camera device or a previously acquired video with a fixed number of frames. A series of transformation nodes are then applied to the original source sequence. Each transformation node performs an operation on the upstream observable sequence that is then passed to downstream nodes. A typical pipeline consists of background subtraction, centroid calculation, tail point calculation, and finally, tail angle calculation. Nodes have a unique set of visualizers that provide the node’s output at each step. Each node has a set of properties associated with the output, such as a single coordinate, an array of coordinates, or an array of angles, which can be used for more sophisticated pipelines. ( |
|
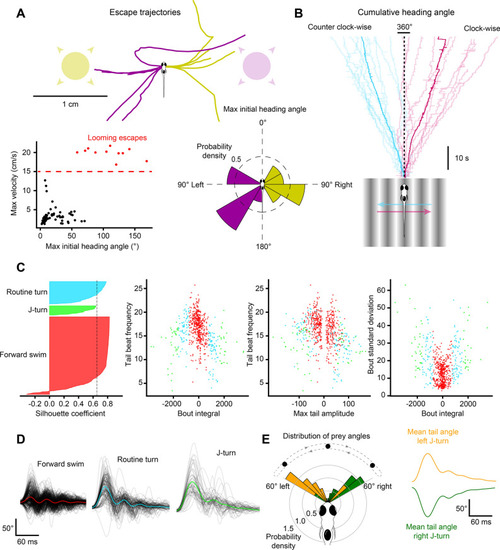
Free-swimming open-loop behavior. Individual freely-swimming zebrafish larva were presented with virtual open-loop visual stimuli while multiple behavioral metrics were recorded (332 Hz). The curvature along 14 tail segments, from the most rostral portion of the tail at the tail base to the most caudal portion of the tail at the tail tip, were calculated and averaged into 4 consecutive bins (cyan to magenta). The angle of the left (red) and right eye (green), the cumulative heading angle (yellow), the visual stimulus angle (black), tail beat frequency (orange), peak amplitudes (navy blue), and bout detection (gray) are displayed. ( |
|
Visual stimulation drives specific behavioral kinematics. ( |
|
Multi-animal tracking during OMR and prey capture. ( |
|
One-dimensional head-fixed closed-loop OMR. The tail of a head-fixed fish was tracked (332 Hz) while presenting a closed-loop OMR stimulus with varying feedback gain values. ( |
|
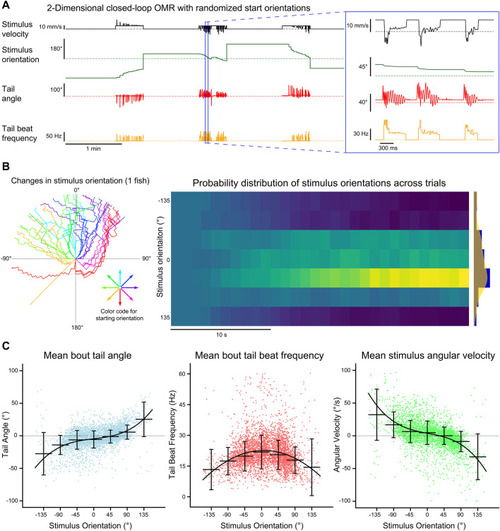
Multi-animal two-dimensional closed-loop OMR. The tails of four head-fixed fish were tracked (332 Hz) while each fish was presented with a two-dimensional closed-loop OMR stimulus. ( |
|
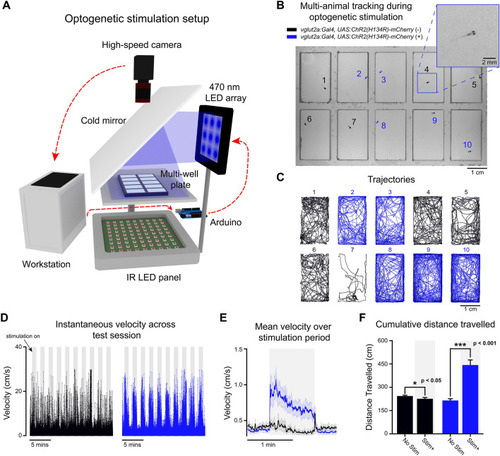
Free-swimming multi-animal tracking during optogenetic stimulation. Ten larvae were tracked simultaneously (200 Hz) in a multi-well plate while being subjected to epochs of optogenetic stimulation. (A) 3D schematic of the optogenetic stimulation setup. Live video is processed by BonZeb, which sends commands to an Arduino to generate a digital signal for triggering a 470 nm LED array (0.9 mW/mm2 at the plate). Components of the optogenetic setup are not to scale. (B) Example of a single video frame. Data are color coded based on genetic background. (C) Example of trajectories over an entire 20-min test session. (D) Instantaneous velocities for all fish plotted across the entire test session. Shaded regions represent periods when optogenetic stimulation occurred. 1-min intervals of stimulation and no stimulation repeated throughout the assay with a 30 s no stimulation period occurring at the beginning and end of the assay. Left: instantaneous velocity of control larvae (n = 15). Right: instantaneous velocity of experimental larvae (n = 15). (E) Mean instantaneous velocity averaged across all fish and all stimulation periods. Instantaneous velocity across each stimulation period was averaged for each fish and convolved with a box car filter over a 1s window. Bold line represents the mean and shaded region represents SEM. (F) Cumulative distance travelled during periods of stimulation ON (Stim +) and stimulation OFF (No stim). |
|
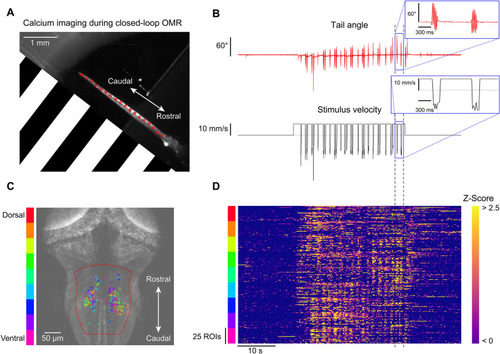
Calcium imaging during closed-loop OMR. One-dimensional closed-loop optomotor gratings were presented to a head-fixed larva while simultaneously performing fast volumetric two-photon calcium imaging. ( |