- Title
-
Spirulina maxima Derived Pectin Nanoparticles Enhance the Immunomodulation, Stress Tolerance, and Wound Healing in Zebrafish
- Authors
- Rajapaksha, D.C., Edirisinghe, S.L., Nikapitiya, C., Dananjaya, S., Kwun, H.J., Kim, C.H., Oh, C., Kang, D.H., De Zoysa, M.
- Source
- Full text @ Mar. Drugs
|
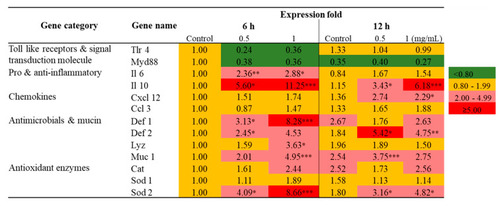
Transcriptional profiling of immune related genes in Raw 264.7 cells exposed to SmPNPs (0.5 and 1 mg/mL) for 6 and 12 h. Data are expressed as means plus or minus standard deviation (± SD) of triplicate samples. Asterisk (*) marks indicate statistical significance compared to SmPNPs treated vs. untreated control (One-way ANOVA * |
|
ROS detoxification effect of SmPNPs on |
|
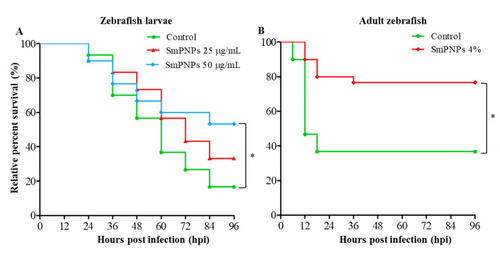
Disease resistance of SmPNPs exposed larvae and adult zebrafish against |
|
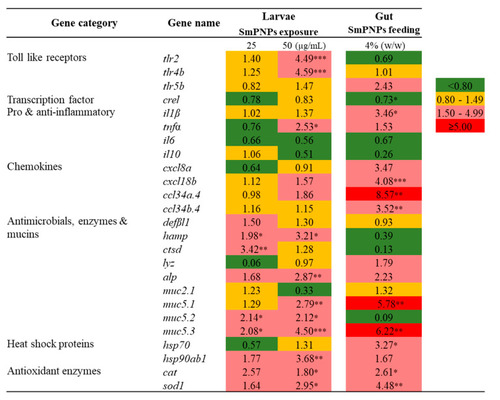
Transcriptional profiling of immune-related genes in zebrafish larvae exposed to SmPNPs (25 and 50 μg/mL) for 5 days and SmPNPs supplemented (4%) diet fed adults for 6 weeks. Data are expressed as means plus or minus standard deviation (± SD) of triplicate samples. Asterisk (*) marks indicate statistical significance compared to SmPNPs treated vs. non-treated control (One-way ANOVA * |
|
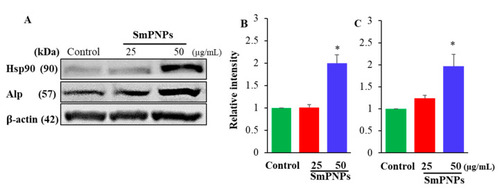
Immunoblotting of heat shock protein 90 (Hsp90) and alkaline phosphatase (Alp) expressions of SmPNPs exposed zebrafish larvae. ( |
|
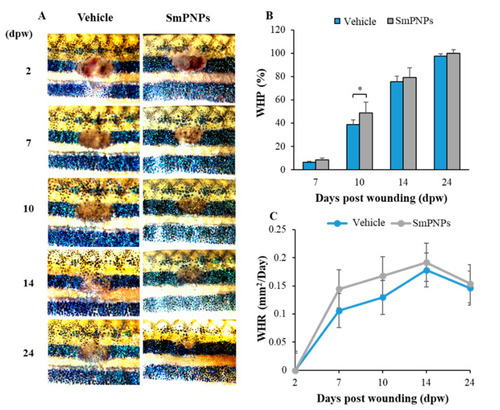
Wound healing effects of SmPNPs on adult zebrafish. ( |
|
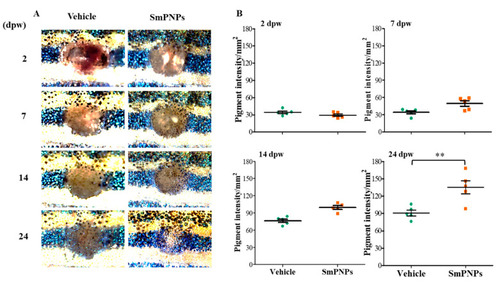
Effect of SmPNPs on pigment restoration during the wound healing. ( |
|
Effect of SmPNPs on wound healing by histology assessment; Hematoxylin and Eosin (H&E) staining of transverse sections of control (unwounded), vehicle (wounded; vehicle treated), SmPNPs (wounded; SmPNPs treated) muscle tissues at 2 and 7 dpw. Normal epidermis (black arrows), dermis and scale (arrowheads), persistent inflammation and thin layer of neoepithelium (blue arrows), completely re-epithelialized with a neoepidermis of multiple cell layer (red arrows) are shown in respective figures. Scale bar × 40 = 500 µm, × 200 = 100 µm. |
|
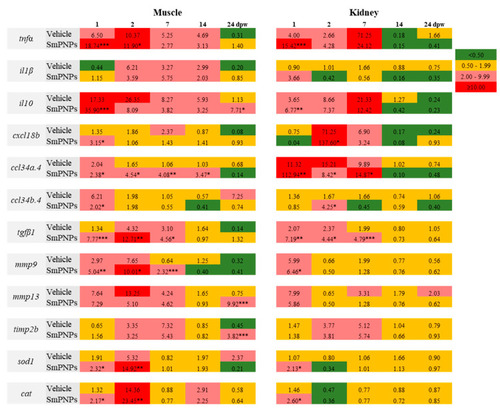
Time-course transcriptional analysis of genes related to wound healing in muscle and kidney of zebrafish. The wounded fish had been treated with vehicle and SmPNPs at different time points (1, 2, 7, 14, and 24 dpw) were analyzed. Data are expressed as means plus or minus standard deviation (± SD) of triplicate samples; (Unpaired two-tailed |