- Title
-
Organophosphorus diisopropylfluorophosphate (DFP) intoxication in zebrafish larvae causes behavioral defects, neuronal hyperexcitation and neuronal death
- Authors
- Brenet, A., Somkhit, J., Hassan-Abdi, R., Yanicostas, C., Romain, C., Bar, O., Igert, A., Saurat, D., Taudon, N., Dal-Bo, G., Nachon, F., Dupuis, N., Soussi-Yanicostas, N.
- Source
- Full text @ Sci. Rep.
|
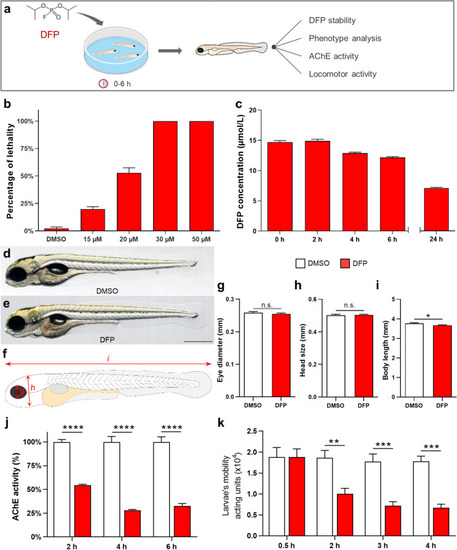
DFP-exposed zebrafish larvae displayed reduced motility and AChE inhibition. ( |
|
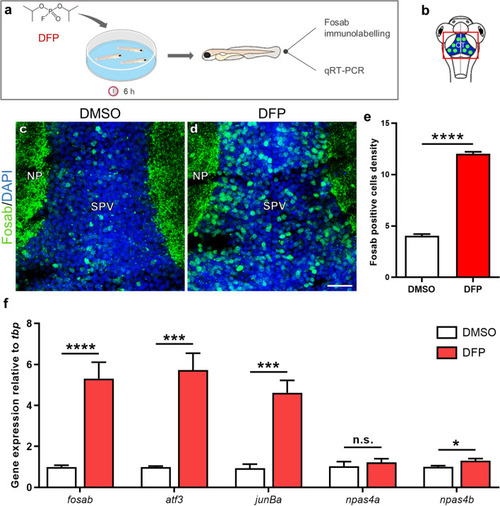
DFP exposure induces overexpression of the IEGs |
|
DFP exposure caused neuronal hyperexcitation. ( |
|
DFP exposure increased cell apoptosis. ( |
|
DFP exposure caused increased NR2B-NMDA subunit receptor accumulation combined with decreased gephyrin and GABA signaling. ( |