- Title
-
Accelerated Amyloid Beta Pathogenesis by Bacterial Amyloid FapC
- Authors
- Javed, I., Zhang, Z., Adamcik, J., Andrikopoulos, N., Li, Y., Otzen, D.E., Lin, S., Mezzenga, R., Davis, T.P., Ding, F., Ke, P.C.
- Source
- Full text @ Adv Sci (Weinh)
|
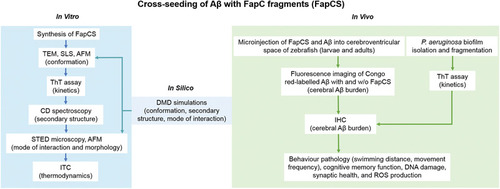
In vitro cross‐seeding between FapC fragments (FapCS) and A |
|
DMD simulations of molecular interactions between FapC and A |
|
FapCS accelerated A PHENOTYPE:
|
|
FapCS accelerated A |
|
|
|
Cross‐seeding of A |