- Title
-
CFTR Depletion Confers Hypersusceptibility to Mycobacterium fortuitum in a Zebrafish Model
- Authors
- Johansen, M.D., Kremer, L.
- Source
- Full text @ Front Cell Infect Microbiol
|
|
|
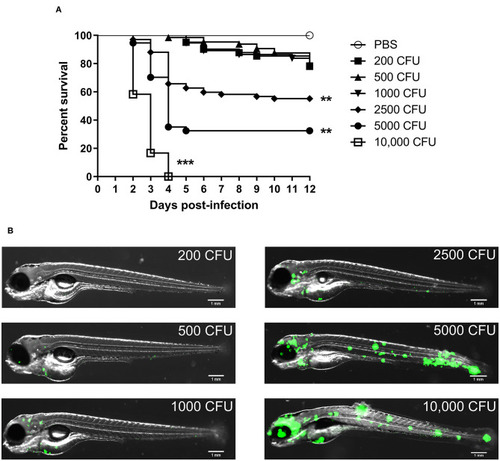
Zebrafish embryos are susceptible to |
|
Zebrafish infected with |
|
Lipoclodronate macrophage depletion results in lethal |
|
CFTR ablation leads to rapid larval death and uncontrolled bacterial expansion. |
|
A hypothetical schematic summarizing the pathogenesis of |