- Title
-
Methiothepin Suppresses Human Ovarian Cancer Cell Growth by Repressing Mitochondrion-Mediated Metabolism and Inhibiting Angiogenesis In Vivo
- Authors
- Lee, J.Y., Yang, C., Lim, W., Song, G.
- Source
- Full text @ Pharmaceutics
|
Methiothepin inhibits the viability and proliferation of ES2 and OV90 cells. ( |
|
Regulation of cell death by methiothepin in ES2 and OV90 cells. ( |
|
Effects of methiothepin on the expression of intrinsic apoptosis regulators in ES2 and OV90 cells. ( |
|
Effect of methiothepin on mitochondrial membrane potential (ΔΨ) and mitochondrial Ca2+ levels in ES2 and OV90 cells. ( |
|
Metabolic profiling following methiothepin treatment in ES2 and OV90 cells. ( |
|
Effects of methiothepin on the expression of ER stress-related proteins in ES2 and OV90 cells. ( |
|
Impairment of vasculogenesis by methiothepin exposure in zebrafish embryos. Fluorescence images of transgenic (fli1:eGFP) zebrafish embryos after treatment with methiothepin for 48 h were captured by fluorescent microscopy. Quantification of fluorescence intensity in the vascular structures of the inner optic circle (IOC), dorsal longitudinal vein (DLV), yolk sac, and caudal vein (CV) of zebrafish embryos following methiothepin exposure was performed using ImageJ software. The asterisks indicate statistically significant differences compared to the control (** |
|
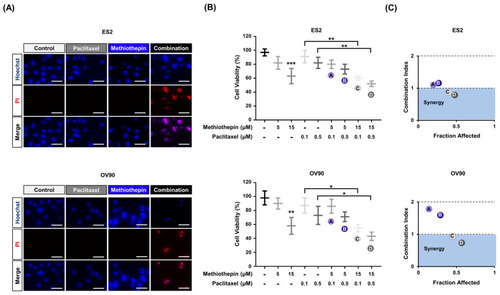
Synergistic effects of methiothepin treatment on paclitaxel sensitivity in ES2 and OV90 cells. ( |
|
Schematic diagram of the methiothepin-associated anti-cancer mechanisms in ovarian cancer cells. In ovarian cancer cells, methiothepin induces depolarization in the mitochondrial membrane (also known as mitochondria membrane potential, ΔΨ) and promotes the influx of Ca2+. Metabolic disorders caused by methiothepin are indicated by a decrease in mitochondrial respiration and adenosine triphosphate (ATP) production. The expression of proteins belonging to the BCL-2 family are inhibited by methiothepin, which leads to the cleavage of poly (ADP-ribose) polymerase (PARP), along with the activation of caspases. Meanwhile, methiothepin increases the expression of ER stress-related proteins and finally induces the expression of C/EBP homologous protein (CHOP), which leads to apoptosis. Methiothepin improves the anti-cancer effect of paclitaxel, greatly reducing the viability of ovarian cancer cells. |