- Title
-
Topological data analysis of zebrafish patterns
- Authors
- McGuirl, M.R., Volkening, A., Sandstede, B.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
Self-organization during development. Diverse skin patterns form on zebrafish due to the interactions of pigment cells. ( |
|
Illustration of persistent homology applied to coordinate data. ( |
|
Illustration of our topological techniques applied to zebrafish patterns. ( |
|
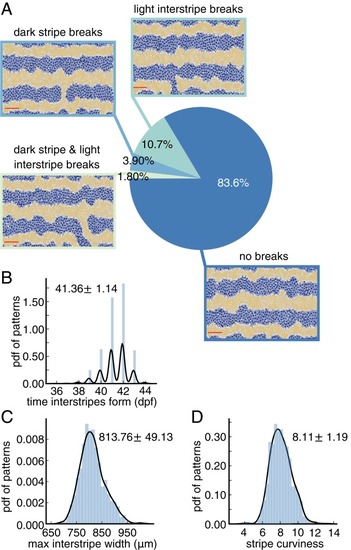
Baseline quantification of wild-type patterns. All measurements are based on |
|
Baseline study of mutant patterns to extract quantifiable features. All measurements are based on |
|
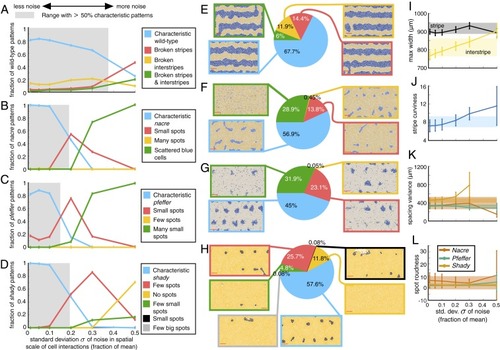
Quantitative study of how stochasticity in cell interactions affects wild-type and mutant zebrafish patterns. For each value of |
|
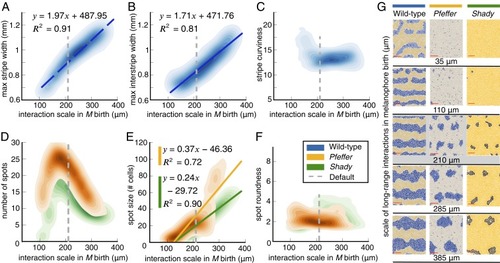
Quantifying in silico pattern dependence on the spatial scale of long-range cellular interactions involved in |