- Title
-
More Favorable Palmitic Acid Over Palmitoleic Acid Modification of Wnt3 Ensures Its Localization and Activity in Plasma Membrane Domains
- Authors
- Azbazdar, Y., Ozalp, O., Sezgin, E., Veerapathiran, S., Duncan, A.L., Sansom, M.S.P., Eggeling, C., Wohland, T., Karaca, E., Ozhan, G.
- Source
- Full text @ Front Cell Dev Biol
|
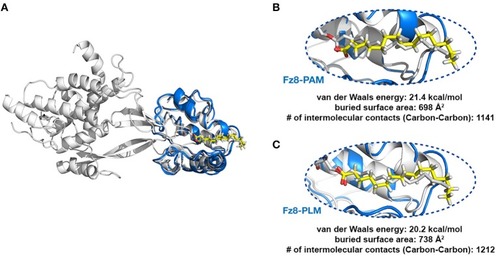
PAM and PLM can adopt a diverse range of conformations. |
|
PLM modified Wnt permits conformationally viable Fz8-PLM interaction. |
|
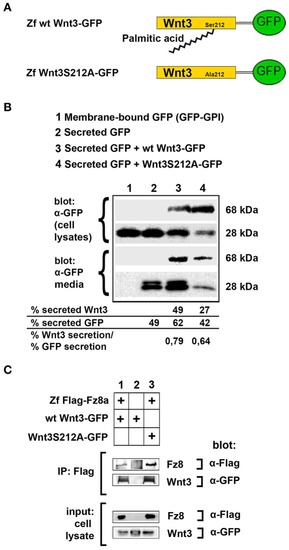
Acylation of Wnt3 at the conserved serine residue (S212) is not necessary for its secretion and interaction with its receptor. |
|
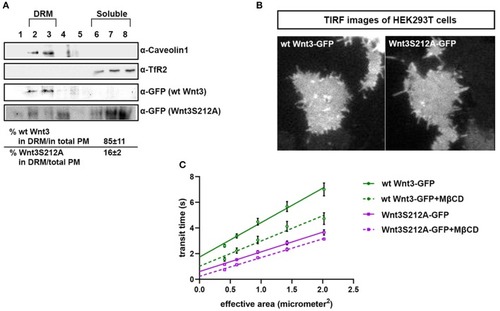
Acylation of Wnt3 facilitates its partitioning into more ordered plasma membrane environments. |
|
Acylation of Wnt3 is essential for activation of canonical Wnt signaling. |